Развитие исследований и разработок в сфере технологий искусственного интеллекта для здравоохранения в Российской Федерации: итоги 2021 года
- Авторы: Гусев А.В.1,2, Владзимирский А.В.3, Шарова Д.Е.3, Арзамасов К.М.3, Храмов А.Е.4,5
-
Учреждения:
- К-Скай
- Центральный научно-исследовательский институт организации и информатизации здравоохранения
- Научно-практический клинический центр диагностики и телемедицинских технологий
- Университет Иннополис
- Балтийский федеральный университет имени Иммануила Канта
- Выпуск: Том 3, № 3 (2022)
- Страницы: 178-194
- Раздел: Обзоры
- Статья получена: 11.05.2022
- Статья одобрена: 11.08.2022
- Статья опубликована: 17.10.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/107367
- DOI: https://doi.org/10.17816/DD107367
- ID: 107367
Цитировать
Аннотация
Применение технологий искусственного интеллекта в российском здравоохранении является одним из приоритетных направлений реализации национальной стратегии развития искусственного интеллекта в нашей стране. Внедрение цифровых решений в медицинских организациях на основе искусственного интеллекта должно способствовать повышению уровня жизни населения и качества медицинской помощи, включая профилактические обследования, диагностику, основанную на анализе изображений, прогнозирование возникновения и развития заболеваний, подбор оптимальных дозировок лекарственных препаратов, сокращение угроз пандемий, автоматизацию и повышение точности хирургических вмешательств и т.д.
Идёт развитие нормативного и технического регулирования в сфере применения искусственного интеллекта в здравоохранении. Создан отечественный рынок соответствующих решений, некоторые из них получили регистрационные удостоверения Росздравнадзора как медицинские изделия. Различными научными коллективами выполняются исследовательские работы. Вместе с этим мы пока существенно отстаём от стран-лидеров в сфере искусственного интеллекта, таких как США и Китай. Инвестиции в продукты искусственного интеллекта для здравоохранения существенно снизились по итогам 2021 г. Основные причины отставания, как минимум с точки зрения рыночных показателей, находятся в низком спросе и ограниченных возможностях государственных медицинских организаций финансировать проекты искусственного интеллекта, а также в сфере доверия к безопасности и эффективности таких решений.
Полный текст
ВВЕДЕНИЕ
В настоящее время применение различных ИТ-решений на основе технологий искусственного интеллекта (ИИ) является одним из самых перспективных направлений цифровой трансформации здравоохранения. Рост интереса к ИИ обусловлен несколькими трендами, включая увеличение аппаратной вычислительной мощности, развитие облачных вычислений, быстрое накопление больших массивов цифровых данных и развитие алгоритмов машинного обучения. Эти предпосылки создали условия для возросших надежд на повышение эффективности работы здравоохранения благодаря внедрению различных ИИ-продуктов, включая улучшение в практической медицине и организации охраны здоровья, направленных, в конечном итоге, на повышение качества медицинской помощи и увеличение эффективности управления ресурсами здравоохранения [1].
Основными направлениями исследований и разработок в сфере программного обеспечения на основе технологий ИИ являются диагностика и прогнозирование развития заболеваний и их осложнений, подбор персонифицированной терапии, работа персональных медицинских помощников для мониторинга и оценки состояния больных в режиме реального времени, разработка новых лекарственных средств и поддержка их клинических испытаний. Отдельным, хотя ещё и недостаточно развитым направлением является создание роботизированных, по-настоящему автономных устройств для сферы здравоохранения.
Потенциально, программное обеспечение на основе ИИ может повысить эффективность работы врачей, медсестёр и медицинских организаций, сокращая время документационного сопровождения процесса оказания медицинской помощи, обеспечивая маршрутизацию пациентов и необходимые коммуникации всех участников процесса. Пандемия COVID-19 существенно усилила интерес к применению ИИ-продуктов. Вместе с этим недавние публикации показали, что для широкого внедрения ИИ-систем в практическом здравоохранении нужны дополнительные исследования и разработки, в том числе для обеспечения независимой клинической проверки и оценки экономической эффективности [2, 3].
По аналитическим данным, объём глобального рынка ИИ для здравоохранения в 2021 г. составил 8,19 млрд долларов, в 2022 г. он вырастет до 10,11 млрд долларов при среднегодовом темпе роста (compound annual growth rate, CAGR) 23,46%, а к 2026 г. его размер увеличится до 49,10 млрд долларов при CAGR 48,44%1.
Инвестиции в ИИ для здравоохранения постоянно растут, начиная с 2017 г. (рис. 1). По данным CB Insights2, в 2021 г. объём суммарных вложений в компании, предлагающие различные продукты на основе технологий ИИ, составил 12,2 млрд долларов (505 сделок). Для сравнения, в 2020 г. эта цифра равнялась 6,627 млрд долларов (397 сделок), в 2019 г. ― 4,129 млрд долларов (367 сделок), а в 2018 г. ― «всего» 2,7 млрд долларов (264 сделки)3.
Рис. 1. Динамика венчурного инвестирования в системы искусственного интеллекта для медицины и здравоохранения, по данным CB Insights, млрд долларов США.
ОСНОВНЫЕ ПРИНЦИПЫ РАЗВИТИЯ РЫНКА ИСКУССТВЕННОГО ИНТЕЛЛЕКТА ДЛЯ ЗДРАВООХРАНЕНИЯ В РОССИЙСКОЙ ФЕДЕРАЦИИ
Основа нормативно-правового регулирования рынка ИИ в Российской Федерации определена Указом Президента РФ № 4904, которым утверждена «Национальная стратегия развития искусственного интеллекта на период до 2030 года».
Целями развития ИИ в Российской Федерации определены рост благосостояния и качества жизни её населения, обеспечение национальной безопасности и правопорядка, достижение устойчивой конкурентоспособности российской экономики, в том числе лидирующих позиций в мире в области ИИ.
Здравоохранение в Стратегии определено как одна из приоритетных отраслей для внедрения ИИ, что должно способствовать достижению стратегических целей и задач национального проекта «Здравоохранение», включая сокращение заболеваемости и смертности, рост ожидаемой продолжительности жизни и т.д.5
Внедрение цифровых решений в медицинских организациях на основе ИИ-технологий должно способствовать повышению уровня жизни населения и качества медицинской помощи, включая профилактические обследования, диагностику, основанную на анализе изображений, прогнозирование возникновения и развития заболеваний, подбор оптимальных дозировок лекарственных препаратов, сокращение угроз пандемий, автоматизацию и повышение точности хирургических вмешательств.
Основными направлениями развития ИИ названы стимулирование спроса и внедрения отечественных продуктов, обеспечение безопасного применения ИИ, совершенствование нормативного и технического регулирования, разработка качественных наборов данных. Для этого на уровне федеральных и региональных органов исполнительной власти должно быть обеспечено решение ряда задач:
- долгосрочная поддержка научных исследований и разработок;
- содействие внедрению программного обеспечения на основе технологий ИИ в государственных органах управления здравоохранением и медицинских организациях;
- развитие образования в сфере ИИ, в том числе повышение уровня информированности населения о возможностях ИИ-технологий;
- развитие нормативного и технического регулирования;
- поддержка экспорта и продвижение российских ИИ-продуктов на международных рынках;
- создание стимулов для привлечения инвестиций в развитие науки, исследований и разработки ИИ-продуктов;
- формирование комплексной безопасности при создании и использовании ИИ-продуктов.
Согласно национальной стратегии развития ИИ, первоочередными задачами являются:
- производство качественных размеченных наборов данных и предоставление контролируемого доступа к ним научным организациям и разработчикам;
- стимулирование создания и развития конкурентного рынка программных продуктов на основе ИИ-технологий в сфере здравоохранения, в том числе создание условий для оплаты использования таких продуктов на всех уровнях оказания медицинской помощи.
Выполнение указанных задач обеспечит рост числа и качества научных исследований и публикаций, значительно повысит объём инвестиций, усилит конкуренцию между разработчиками, а в итоге улучшит качество программных решений на основе ИИ-технологий для здравоохранения, увеличит объёмы и повысит эффективность их применения.
Основными показателями, характеризующими успех реализации ИИ-стратегии в сфере здравоохранения, является увеличение:
- числа компаний-разработчиков ИИ-продуктов для медицины и здравоохранения;
- количества результатов интеллектуальной деятельности (патенты, публикации в российских и международных научных рецензируемых журналах и т.д.);
- числа продуктов, которые прошли государственную регистрацию, в том числе как программные медицинские изделия;
- числа государственных органов и организаций в сфере здравоохранения, использующих продукты на основе ИИ для повышения эффективности своей деятельности;
- количества и индекса цитируемости в ведущих мировых изданиях научных статей российских учёных на тему ИИ в здравоохранении;
- числа доступных наборов данных, размеченных и верифицированных квалифицированными специалистами-медиками.
Развитие нормативного регулирования
К базовым принципам и задачам нормативно-правового регулирования ИИ, которые определены Распоряжением Правительства РФ № 2129-р6, относятся создание механизмов упрощённого внедрения программного обеспечения, созданного с использованием технологий ИИ, установление юридической ответственности при применении такого программного обеспечения, развитие страховых институтов, совершенствование режима оборота данных, создание национальной системы технического регулирования и оценки соответствия, разработка комплекса мер по стимулированию развития технологий.
С точки зрения внедрения ИИ, отрасль здравоохранения является особым случаем: для неё вопросы безопасности, эффективности и доверия к применению ИИ-решений в реальной клинической практике являются краеугольными.
Нормативное регулирование разработки, вывода на рынок и применения ИИ-продуктов в сфере здравоохранения развивается по двум ключевым направлениям:
- обеспечение безопасности, качества и эффективности ИИ-продуктов как программных медицинских изделий;
- обеспечение мер по защите данных и конфиденциальности, включая кибер-безопасность и оборот обезличенных медицинских данных для целей машинного обучения.
В случае если программное обеспечение, созданное на основе ИИ-технологий, предназначено производителем для лечебно-диагностических целей (при оказании медицинской помощи), оно относится к медицинским изделиям. Правовой основной для этого является Федеральный закон № 4-ФЗ7, которым Российская Федерация ратифицировала «Соглашения о единых принципах и правилах обращения медицинских изделий (изделий медицинского назначения и медицинской техники) в рамках Евразийского экономического союза». Документом преду-смотрено, что выработка общей скоординированной политики обращения медицинских изделий должна быть основана на правилах и рекомендациях добровольной группы регуляторов в области медицинских изделий со всего мира (International Medical Device Regulators Forum, IMDRF), которая объединила свои усилия для развития и гармонизации руководящих принципов [4]. В составе IMDRF создана Рабочая группа по медицинским изделиям на основе ИИ (AIMD), целью которой является достижение согласованного подхода к управлению данным видом медицинских изделий. Её деятельность охватывает стандартизацию и выработку единых нормативно-технических подходов для медицинских изделий, основанных на машинном обучении.
Начиная с осени 2019 г. при Росздравнадзоре ведёт свою деятельность специальная рабочая группа, которая рассматривает предложения по изменению действующих нормативно-правовых актов Российской Федерации и созданию новых документов для регулирования разработки и вывода на рынок программного обеспечения на основе ИИ, базируясь на рекомендациях и регуляторных документах IMDRF.
В соответствии с Федеральным законом № 323-ФЗ8, обращение медицинских изделий, включая программные медицинские изделия, разрешено только при условии предварительной государственной регистрации в установленном законом порядке.
До 2022 г. регистрация программного обеспечения, созданного на основе технологий ИИ, в России осуществлялась по национальным правилам. Для этого Росздравнадзор совместно с Министерством здравоохранения Российской Федерации внесли все необходимые изменения в действующие нормативно-правовые акты, регулирующие оборот медицинских изделий, с учётом особенностей ИИ. В частности, были выпущены новые версии следующих документов:
- Постановление Правительства РФ от 27.12.2012 № 1416 «Об утверждении Правил государственной регистрации медицинских изделий»9, с изменениями и дополнениями, внесёнными Постановлением Правительства РФ от 24.11.2020 № 1906 «О внесении изменений в Правила государственной регистрации медицинских изделий»10;
- Приказ Минздрава РФ от 19.01.2017 № 11н «Об утверждении требований к содержанию технической и эксплуатационной документации производителя (изготовителя) медицинского изделия»11;
- Приказ Минздрава РФ от 30.08.2021 № 885н «Об утверждении Порядка проведения оценки соответствия медицинских изделий в форме технических испытаний, токсикологических исследований, клинических испытаний в целях государственной регистрации медицинских изделий»12;
- Приказ Минздрава РФ от 06.06.2012 № 4н «Об утверждении номенклатурной классификации медицинских изделий»13, с изменениями и дополнениями, внесёнными Приказом Минздрава РФ от 07.07.2020 № 686н «О внесении изменений в приложения № 1 и № 2 к Приказу Министерства здравоохранения Российской Федерации от 06.06.2012 № 4н «Об утверждении номенклатурной классификации медицинских изделий»»14.
Начиная с 1 января 2026 г. регистрация медицинских изделий будет осуществляться только по правилам Евразийского экономического союза (ЕАЭС). Для этого внесены необходимые изменения в нормативно-правовые акты Евразийской экономической комиссии (ЕЭК), в частности в рекомендации Коллегии ЕЭК от 12.11.2018 № 2515, где предусмотрен отдельный раздел № 7 «Программное обес-печение».
Развитие технического регулирования
В настоящее время в России одновременно со всеми ведущими странами мира в части внедрения ИИ (США, Китай, Канада, Южная Корея, страны Евросоюза) приняты национальные стратегии или иные документы, устанавливающие национальные цели, задачи и планы развития ИИ [5]. Данные документы закрепляют основополагающие принципы и подходы к регулированию ИИ-технологий. На основе национальных стратегий создаются нормативно-правовые акты и технические стандарты.
Начиная с 2019 г. в России запущен собственный процесс разработки и утверждения национальных технических стандартов в сфере ИИ для медицины и здравоохранения. Основные цели этой работы заключаются в содействии социально-экономическому развитию нашего государства, интеграции в мировую экономику и международные системы стандартизации, повышении уровня качества решений и услуг и увеличении их конкурентоспособности.
Приказом Федерального агентства по техническому регулированию и метрологии (Росстандарт) № 173216 в июле 2019 г. создан технический комитет по стандартизации «Искусственный интеллект» (ТК 164), который занимается вопросами ИИ и повышения эффективности работ по стандартизации в данной области [6]. В рамках ТК 164 учреждён отдельный специальный подкомитет 01 «Искусственный интеллект в здравоохранении» (ПК 01), разрабатывающий национальные и международные стандарты и действующий в целях координации работ по унификации и стандартизации требований к разработке, тестированию и эксплуатации систем ИИ в здравоохранении, а также в целях установки сертификационных требований к медицинским изделиям, использующим технологии ИИ (приказ Росстандарта от 31.12.2019 № 347117). ПК 01 действует на базе ГБУЗ г. Москвы «Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения города Москвы». В состав подкомитета входят 24 профильных организации и независимые внешние эксперты. Члены ПК 01, в соответствии с планом деятельности ТК 164, постоянно работают над созданием национальных стандартов. За 2021 г. были разработаны первые 6 документов из серии «Системы искусственного интеллекта в клинической медицине», в том числе «Клинические испытания», «Программа и методика технических испытаний», «Управление изменениями в системах искусственного интеллекта с адаптивными алгоритмами», «Оценка и контроль эксплуатационных параметров», «Требования к структуре и порядку применения набора данных для обучения и тестирования алгоритмов», «Общие требования к эксплуатации» (табл. 1) [7].
Таблица 1. Список действующих национальных стандартов Российской Федерации в сфере искусственного интеллекта для медицины
№ | Название и реквизиты стандарта |
1 | Национальный стандарт РФ ГОСТ Р 59921.1-2022 «Системы искусственного интеллекта в клинической медицине. Часть 1. Клиническая оценка» |
2 | Национальный стандарт РФ ГОСТ Р 59921.2-2021 «Системы искусственного интеллекта в клинической медицине. Часть 2. Программа и методика технических испытаний» |
3 | Национальный стандарт РФ ГОСТ Р 59921.3-2021 «Системы искусственного интеллекта в клинической медицине. Часть 3. Управление изменениями в системах искусственного интеллекта с непрерывным обучением» |
4 | Национальный стандарт РФ ГОСТ Р 59921.4-2021 «Системы искусственного интеллекта в клинической медицине. Часть 4. Оценка и контроль эксплуатационных параметров» |
5 | Национальный стандарт РФ ГОСТ Р 59921.5-2022 «Системы искусственного интеллекта в клинической медицине. Часть 5. Требования к структуре и порядку применения набора данных для обучения и тестирования алгоритмов» |
6 | Национальный стандарт РФ ГОСТ Р 59921.6-2021 «Системы искусственного интеллекта в клинической медицине. Часть 6. Общие требования к эксплуатации» |
Выпущенные стандарты не имеют аналогов в мире. Согласно перспективному плану работы до 2025 г., ТК 164 должен координировать разработку около 50 стандартов в области ИИ в здравоохранении. Кроме этого, выпущен ряд отдельных методических рекомендаций, которые также можно использовать в качестве инструментов технического регулирования (табл. 2).
Таблица 2. Список методических рекомендаций по теме систем искусственного интеллекта для здравоохранения
№ | Название и реквизиты документа |
1 | Регламент подготовки наборов данных с описанием подходов к формированию репрезентативной выборки данных. Часть 1: методические рекомендации / сост. С.П. Морозов, А.В. Владзимирский, А.Е. Андрейченко, и др. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 103. Москва: ГБУЗ НПКЦ ДиТ ДЗМ, 2021. 40 с. |
2 | Клинические испытания программного обеспечения на основе интеллектуальных технологий (лучевая диагностика). К-49 Морозов С.П., Владзимирский А.В., Кляшторный В.Г., и др. Клинические испытания программного обеспечения на основе интеллектуальных технологий (лучевая диагностика). Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 57. Москва, 2019. 51 с. |
3 | Методические рекомендации по порядку проведения экспертизы качества, эффективности и безопасности медицинских изделий (в части программного обеспечения) для государственной регистрации в рамках национальной системы. Москва: ФГБУ ВНИИИМТ Росздравнадзора, 2021. 33 с. |
ОБЗОР РОССИЙСКИХ РЕШЕНИЙ В СФЕРЕ ИСКУССТВЕННОГО ИНТЕЛЛЕКТА ДЛЯ ЗДРАВООХРАНЕНИЯ
Согласно концепции S-образной кривой развития, в настоящее время российский рынок ИИ для здравоохранения переходит от этапа формирования к этапу быстрого роста. Насколько быстро осуществится этот переход, зависит не только от разработчиков ИИ-технологий, но и от скорости актуализации нормативно-правовых актов, создания механизмов финансирования медицинских услуг, оказываемых с применением программного обеспечения на основе ИИ.
П.А. Комарь и соавт. [8] разработали рейтинг российских ИИ-стартапов (от англ. start up ― запускать), предлагающих различные продукты для медицины и здравоохранения, основанный на многофакторной методике оценки, включая рыночные перспективы, метрики интереса инвесторов, данные об интеллектуальной собственности и т.д. Согласно данному рейтингу, на российском рынке ИИ-решений для здравоохранения присутствует более 30 различных компаний, из них свыше 60% работают в секторе анализа медицинских изображений, включая такие продукты, как «Третье мнение», Botkin.Ai и Celsus. Для сравнения, в мире насчитывается порядка 2,8 тыс. компаний, работающих в сфере ИИ для медицины и здравоохранения, таким образом, доля российского сегмента в глобальном рынке по числу компаний составляет порядка 1,25%.
Вместе с тем к механическому подсчёту количества компаний нужно относиться критично. В глобальной перспективе приток инвестиций часто приводит к взрывному росту предпринимательской активности ― появлению многочисленных стартапов, ориентированных только на временное существование «на волне хайпа» за счёт поддержки инвесторов, а не на создание реальных продуктов. Стратегически более правильно наблюдать за динамикой выхода на рынок продуктов, регистрацией их в качестве медицинских изделий, объёмами применения и выручки за счёт реализации своих разработок (а не за счёт инвестиций). Таким образом, можно выявить процесс перехода количества в качество.
На конец 2021 г. 7 (20%) из 35 компаний получили суммарно 11 регистрационных удостоверений Росздравнадзора на свои продукты как медицинские изделия, воспользовавшись одноэтапной процедурой регистрации, предусмотренной Постановлением Правительства № 190618. Первым продуктом, созданным с применением технологий ИИ и получившим при этом регистрационное удостоверение, стала платформа прогнозной аналитики Webiomed, которая прошла соответствующую государственную регистрацию весной 2020 г. Затем, в ноябре и декабре 2020 г. ещё 2 продукта в сфере анализа медицинских изображений с использованием ИИ также получили регистрационные удостоверения: платформа для анализа и обработки медицинских изображений Botkin.Ai и сервисная платформа лучевой диагностики Care Mentor Ai соответственно (табл. 3).
Таблица 3. Список выданных Росздравнадзором регистрационных удостоверений на программные медицинские изделия, созданные с применением технологий искусственного интеллекта, по состоянию на декабрь 2021 г.
№ | Дата выдачи | Данные о регистрационном удостоверении, продукте и производителе |
1 | 03.04.2020 | Программное обеспечение «Система для поддержки принятия врачебных решений WEBIOMED по ТУ 62.01.29-001-12860736-2019», РУ № РЗН 2020/9958, разработчик «К-Скай», сайт: https://webiomed.ai/ |
2 | 03.11.2020 | Обеспечение программное прикладное Botkin.АI для визуализации и обработки изображений стандарта DICOM по ТУ 58.29.32-001-45146066-2020, РУ № РЗН 2020/12028, разработчик «Интеллоджик», сайт: https://botkin.ai/ |
3 | 11.12.2020 | Программное обеспечение «Система нейросетевая Care Mentor АI» по ТУ 62.01.29-001-28263422-2019, варианты исполнения: Webshow, API. РУ № РЗН 2020/11137, разработчик «КэреМенторЭйАй», сайт: https://carementor.ru/ |
4 | 27.05.2021 | Программное обеспечение «Система нейросетевая Care Mentor AI для диагностики новой коронавирусной инфекции COVID-19 по данным компьютерной томографии» по ТУ 58.29.32-002-28263422-2020, варианты исполнения: Webshow, API. РУ № РЗН 2021/14406, разработчик «КэреМенторЭйАй», сайт: https://carementor.ru/ |
5 | 27.05.2021 | Программное обеспечение ЦЕЛЬС® (ПО ЦЕЛЬС®) по ТУ 58.29.32-001-28139219-2019, РУ № РЗН 2021/14449, разработчик «Медицинские скрининг системы», сайт: https://celsus.ai |
6 | 01.06.2021 | Программный модуль для анализа флюорограмм и рентгенограмм грудной клетки человека по ТУ 58.29.32-001-21494354-2020, РУ № РЗН 2021/14506, разработчик «ПТМ», сайт: https://thirdopinion.ai/ |
7 | 27.07.2021 | Программное обеспечение «Система нейросетевая Саrе Mentor AI для анализа рентгеновской проекционной маммографии» по ТУ 58.29.32-003-28263422-2021, варианты исполнения: Webshow, API. РУ № РЗН 2021/14869, разработчик «КэреМенторЭйАй», сайт: https://carementor.ru/ |
8 | 12.10.2021 | Программное обеспечение «Система нейросетевая Care Mentor AI для определения продольного плоскостопия по данным боковой рентгенографии стопы под нагрузкой» по ТУ 58.29.32-004-28263422-2021, варианты исполнения: Webshow, API. РУ № РЗН 2021/15554, разработчик «КэреМенторЭйАй», сайт: https://carementor.ru/ |
9 | 22.06.2021 | Комплекс программный для автоматической обработки радиологических изображений «Платформа RADLogics» по ТУ 58.29.32-320-17493389-2020, РУ № РЗН 2021/14627, разработчик «Радлоджикс Рус», сайт: https://www.radlogics.com/ |
10 | 24.09.2021 | Программный модуль для анализа исследований компьютерной томографии человека по ТУ 58.29.32-002-21494354-2021, РУ № РЗН 2021/14651, разработчик «ПТМ», сайт: https://thirdopinion.ai/ |
11 | 27.11.2021 | Комплекс программ для регистрации, визуализации, обработки, архивирования и передачи медицинских изображений и данных «Гамма Мультивокс» по ТУ 62.01.29-001-16428326-2018, РУ № РЗН 2021/13277, сайт: https://www.gammamed.ru/ |
НАУЧНЫЕ ИССЛЕДОВАНИЯ В СФЕРЕ ИСКУССТВЕННОГО ИНТЕЛЛЕКТА В ЗДРАВООХРАНЕНИИ
Ярким, но практически забытым эпизодом развития технологий ИИ в России можно считать книгу «Начертание нового способа исследования при помощи машин, сравнивающих идеи», изданную в 1832 г. ветераном наполеоновских войн, чиновником Министерства внутренних дел Семёном Николаевичем Корсаковым (1787–1853). В этом труде, основанном на принципах механистического материализма, автор предложил конструкции пяти «машин, сравнивающих идеи», перфокарты, метод многокритериального поиска с использованием весовых коэффициентов и первый способ обработки больших данных (предтеча современных алгоритмов ИИ) [9].
Официальным началом разработок и исследований в сфере ИИ в нашей стране стал прошедший в 1954 г. междисциплинарный семинар «Автоматы и мышление» под руководством академика А.А. Ляпунова [10]. До конца 80-х годов прошлого столетия уровень теоретических исследований в СССР в области ИИ как минимум соответствовал мировым трендам, а разработки в сфере автоматизированного анализа медицинских данных (в частности, труды Ю.И. Неймарка и А.П. Матусовой в сфере математической обработки электрокардиосигналов) их явно опережали. В 1974 г. в Комитете по системному анализу при Президиуме АН СССР был создан научный совет по проблеме ИИ, который поддерживал работы в области обработки естественного языка, представления знаний и данных, интеллектуального поведения роботов и т.д. Однако с 80-х годов наблюдается постепенное отставание в прикладных технологиях ИИ и машинного обучения, вызванное ухудшением социально-экономической ситуации и последующим распадом СССР. Впрочем, в сфере здравоохранения нужно учитывать ещё один фактор стагнации разработок в сфере ИИ в период 1980–1990-х годов, когда было предложено изрядное количество «экспертных систем» (фактически автоматизированных систем поддержки принятия врачебных решений) для кардиологии, хирургии, эндокринологии, нейрохирургии и т.д. Однако масштабного эффекта они не произвели. В основе этих разработок лежали структурированные «деревья решений» (они могли быть очень обширными, но предсказуемыми и полностью статичными); как такового обучения и развития алгоритмов в процессе эксплуатации не происходило. Для врачей такое программное обеспечение не представляло практической значимости, интерес к автоматизированному анализу медицинских данных резко снизился.
Своим возрождением в начале 2000-х годов ИИ обязан новому математическому аппарату (в том числе искусственным нейронным сетям), стремительному наращиванию вычислительных мощностей, масштабному накоплению биомедицинских данных в цифровом виде.
Согласно «Национальной стратегии развития искусственного интеллекта на период до 2030 года»19, одним из приоритетов развития ИИ в России является развитие научных исследований и разработок в этой сфере, в том числе в здравоохранении. На рис. 2 проиллюстрирована динамика числа научных публикаций российских авторов в международных научных журналах и авторитетных научных конференциях, индексируемых в базе данных научной информации Scopus, тематика которых соответствует применению технологий ИИ в медицине и здравоохранении. Хорошо виден постоянный рост с 2016 г. В 2021 г. число публикаций, находимых по приводимому в подрисуночной подписи запросу, составило 227 статей и обзоров. Анализ публикаций показал, что более 60% работ были сделаны совместно с зарубежными коллегами. Этот факт можно только приветствовать, так как он свидетельствует о расширении международного научного сотрудничества российских исследователей и врачей в такой бурно развивающейся области.
Рис. 2. Число индексируемых публикаций в базе научной информации Scopus, которые были опубликованы российскими авторами на стыке медицины и искусственного интеллекта за последние 10 лет.
Примечание. Запрос в базу данных: (TITLE-ABS-KEY (medicine OR healthcare) AND («artificial intelligence» OR «machine learning») AND AFFILCOUNTRY (Russia OR «Russian Federation»)).
Вместе с тем следует отметить, что число опубликованных результатов исследований мирового уровня в области медицинского ИИ всё же остаётся относительно небольшим по сравнению со странами-лидерами в этой области. Так, аналогичный запрос в Scopus показывает, что в 2021 г. китайскими исследователями было опубликовано 1926 статей, авторами из Великобритании ― 1661, из США ― 4087. Это говорит об отставании, которое необходимо преодолевать опережающими темпами развития в сфере исследований и разработок по системам медицинского ИИ в Российской Федерации. Несомненно, требуются не только инвестиции в разработку, но и дополнительное целевое финансирование исследователей из медицинских научных центров, клинических учреждений.
Показательно, что большинство работ российских авторов в 2021 г. финансировались государственными органами поддержки науки ― Российским фондом фундаментальных исследований (38 статей), Министерством образования и науки Российской Федерации (20 статей) и Российским научным фондом (13 статей). Среди финансирующих организаций упоминаются также российские университеты, но практически нет опубликованных результатов исследований, профинансированных частными российскими инвесторами или бизнесом. Это подтверждает низкий интерес бизнеса к инвестициям в исследования и разработки в области ИИ в медицине в России в последние несколько лет, а также преобладающее государственное финансирование исследований в этой области (необходимо учитывать, что в 2021 г. в основном были опубликованы результаты исследований, проведённых в предыдущие годы, учитывая продолжительное время подготовки, рецензирования и издания статьи в ведущих журналах).
Цели и содержание российских исследований в сфере медицинского ИИ во многом следуют мировым тенденциям. Большое число работ в 2021 г. было посвящено пилотным проектам по изучению возможностей ИИ в рамках различных аспектов помощи пациентам с диагнозом новой коронавирусной инфекции COVID-19. На 38-й Международной конференции по машинному обучению (International Conference on Machine Learning, ICML, 2021 г.) лаборатория по ИИ Сбербанка представила два ИИ-сервиса: модель по определению очагов пневмонии на рентгеновских снимках c дальнейшей приоритизацией пациентов для лечения и модель, позволяющую оценивать риск тяжёлого течения заболеваний у пациентов, госпитализированных с пневмонией, в том числе вызванной COVID-1920. Модель по определению очагов пневмонии на рентгеновских снимках достигла точности 97,8% благодаря постоянному обучению и улучшению параметров.
ФГАОУ ВО «Национальный исследовательский Томский политехнический университет» совместно ФГБНУ «Научно-исследовательский институт комплексных проблем сердечно-сосудистых заболеваний» и EPAM Systems (Санкт-Петербург), а также рядом американских коллег из различных компаний и университетов выполнена разработка в сфере применения ИИ для диагностики пневмонии, в том числе связанной с COVID-19 [11]. Авторы показали, что стандартные подходы к глубокому обучению, применяемые в большинстве работ по диаг-ностике заболеваний лёгких, не активируются вокруг паттернов, указывающих на COVID-19 или пневмонию. Было предложено обучение нейросети, основанное на непрямом наблюдении, которое обеспечило точность, сравнимую с точностью специализированных сетей, созданных для диагностики COVID-19 и пневмонии. Авторы показали, что предобученная свёрточная сеть VGG-16 [12], дообученная с помощью управляемого внимания, продемонстрировала наиболее точную классификацию на уровне 88 и 84% на валидационном и тестовом подмножествах соответственно.
В Университете Иннополис (Казань) в рамках Института искусственного интеллекта также была разработана система выявления COVID-19 c помощью ИИ на основе рентгеновских снимков. Для обучения ИИ в набор данных включили 28 тыс. снимков лёгких здоровых людей и пациентов с разными видами пневмонии, а также 94 рентгеновских изображения органов грудной полости, заражённых коронавирусом. Итогом исследования стала нейросеть, которая диагностировала коронавирус по рентгеновским снимкам с точностью до 80%: так нейронная сеть научилась определять общие признаки патологий, вызванных COVID-19.
Дополнительно укажем, что в сфере решений для лучевой диагностики активно работает платформа Botkin.AI. Решение легко интегрируется с медицинскими информационными системами, обеспечивает автоматизированный отбор, деперсонализацию и обработку медицинских снимков с помощью ИИ. С 2017 г. компания работает с ведущими фармкомпаниями, частными и государственными клиниками (более 30 успешных проектов в регионах России, странах СНГ, Латинской Америки и Ближнего Востока). На данный момент основные решения компании связаны с автоматизированным анализом результатов маммографии, компьютерной томографии органов грудной клетки и головного мозга.
Конечно, работы российских исследователей в области систем ИИ для медицины не ограничиваются проблематикой COVID-19. В ФГБУ «Национальный медицинский исследовательский центр глазных болезней имени Гельмгольца» Минздрава России (Москва) изучается применение методов ИИ для диагностики диабетической ретинопатии по снимкам глазного дна [13].
Применение машинного обучения в решении прогностических задач, в том числе в части профилактики сердечно-сосудистых заболеваний, изучается на Дальнем Востоке под руководством К.И. Шахгельдян [14].
Исследования и разработки в сфере прогнозирования хронических неинфекционных заболеваний, а также исследования данных реальной клинической практики на основе технологий ИИ проводятся в рамках разработки платформы Webiomed [15]. Платформа предназначена для автоматического анализа обезличенных медицинских данных с целью прогнозирования возможного развития заболеваний и их осложнений на персональном и популяционном уровнях. Поддерживается анализ свыше 2,7 тыс. различных признаков, которые извлекает с помощью NLP-технологий из врачебных протоколов и других документов система электронных медицинских карт пациента. Возможен автоматизированный анализ по 40 различным заболеваниям, включая 18 прогнозных и диагностических моделей машинного обучения, точность некоторых из них превышает 80%. Платформа Webiomed ― пока что единственное решение в Российской Федерации по анализу данных электронных медицинских карт.
Вопросами построения систем объяснимого ИИ в медицине применительно к диагностике заболеваний центральной нервной системы занимается ФГБУ «Национальный медико-хирургический центр имени Н.И. Пирогова» Минздрава России (Москва) совместно с Центром нейротехнологий и искусственного интеллекта Балтийского федерального университета имени Иммануила Канта (Калининград) и лабораторией нейронауки и когнитивных технологий Университета Иннополис (Казань). В частности, в 2021 г. была разработана система разметки данных эпилептической электроэнцефалографии на основе обучения без учителя, базируясь на представлении эпилептических разрядов с использованием теории экстремальных событий [16]. Созданная система поддержки принятия врачебных решений, разработанная резидентом Сколково ООО «Иммерсмед», в настоящее время проходит клиническую апробацию в Пироговском центре [17]. Коллективом предложены также методы на основе ИИ для диагностики возрастных нейродегенративных изменений по функциональным моторным и когнитивным пробам [18].
Использование методов машинного обучения для анализа биоэлектрических сигналов востребовано при реализации технологий интерфейсов мозг-компьютер для задач реабилитации пациентов после черепно-мозговых травм и инсультов [19]. Одним из подходов к реабилитации после инсульта является тренировка посредством мысленного представления движения в контуре интерфейса мозг-компьютер, позволяющая контролировать результат каждой попытки представления движения.
Самым крупным в России научным проектом в сфере ИИ в здравоохранении является московский эксперимент по применению технологий компьютерного зрения в лучевой диагностике, запущенный в 2020 г. при поддержке Правительства Москвы в виде мультицентрового проспективного исследования целесообразности применения технологий ИИ в условиях реальной клинической практики (www.mosmed.ai). В исследовании приняли участие 18 компаний-разработчиков; в Единый радиологический информационный сервис (ЕРИС ЕМИАС) интегрировано 53 ИИ-сервиса для анализа результатов рентгенографий, компьютерной томографии, маммографий. За 2 года выполнен автоматизированный анализ свыше 5,6 млн лучевых исследований, результаты которого доступны более чем 10 тыс. врачей 150 медицинских организаций. Осуществляется комплексный научный анализ вовлечённости пользователей, технологического качества и диагностической точности алгоритмов ИИ, их влияния на эффективность работы врачей и медицинских организаций. Уже сейчас доказано снижение длительности описаний результатов некоторых видов лучевых исследований благодаря предварительному автоматизированному анализу. Однозначно установлена целесообразность применения ИИ-сервисов в лучевой диагностике для повышения производительности труда врачей-рентгенологов. В целях развития рынка ИИ в рамках эксперимента ведётся постоянная работа с компаниями-участниками, в том числе в формате семинаров, встреч с врачами. В целях выполнения положений Национальной стратегии развития ИИ в Российской Федерации создаётся библиотека наборов данных [20]. Материалы эксперимента послужили основой первых национальных стандартов, указанных выше, и методик клинических испытаний, проводимых в процессе регистрации программного обеспечения на основе ИИ в качестве медицинского изделия.
Ежемесячно формируется рейтинг ИИ-сервисов ― участников эксперимента; в его основе лежит совокупность метрик технологического качества и диагностической точности. По каждому направлению эксперимента в открытом доступе публикуется по три лидера, из числа наиболее постоянных участников такого лидерборда (от англ. Leaderboard ― доска лидеров) можно указать несколько разработок, описанных далее.
ИИ-сервис «Третье Мнение» ― одна из первых компаний, заявивших о себе на российском рынке интеллектуальных технологий для здравоохранения. Ведутся разработки алгоритмов для распознавания оцифрованных мазков клеток крови и костного мозга, лучевых исследований органов грудной клетки, а также снимков глазного дна. Компания «Третье Мнение» одной из первых на российском медтех-рынке предложила модуль непрерывного ИИ-анализа видеопотока из больничных учреждений с целью предотвращения образования пролежней, падений пациентов и других больничных происшествий. Впоследствии сервис установили в нескольких ведущих частных медицинских центрах страны. ИИ-сервис платформы «Третье Мнение» для автоматизированного анализа результатов рентгенографии органов грудной клетки достаточно стабильно входит в тройку лидеров соответствующего направления эксперимента. При проспективной оценке диагностической точности сервис достигает значения площади под характеристической кривой 0,84 (95% ДИ 0,83–0,85).
ИИ-сервис Celsus ― уверенный участник лидерборда эксперимента по направлению «маммография». При проспективной оценке диагностической точности сервис достигает значения площади под характеристической кривой 0,74 (95% ДИ 0,73–0,74). Отметим, что платформа Celsus поддерживает работу по четырём модальностям: маммография, флюорография, рентгенография органов грудной клетки, компьютерная томография органов грудной клетки и головного мозга. Однако в эксперименте наиболее высокие результаты связаны именно с автоматизированным анализом результатов профилактических лучевых исследований молочных желёз.
ИИ-сервис IraLabs отличается глубокой методологической проработкой проблем автоматизированной обработки результатов лучевых исследований, а также публикационной активностью. В рамках эксперимента компания предоставляет сразу несколько сервисов для оппортунистического скрининга хронических неинфекционных заболеваний, остеопороза, а также оценки объёма поражений лёгких при COVID-19. Наиболее стабильное участие компании в лидерборде эксперимента связано именно с последним направлением: точность алгоритма для работы с проявлениями новой коронавирусной инфекции на компьютерных томограммах органов грудной клетки (площадь под характеристической кривой) ― 0,88 (95% ДИ 0,87–0,88). В скрининге остеопороза ИИ-сервис этого разработчика очень быстро достиг хорошего результата ― 0,94 (95% ДИ 0,88–1,00).
Необходимо подчеркнуть, что приводимые данные не являются полным или официальным обзором лидеров рейтинга. Это лишь акцент на нескольких активных участниках рынка, развивающих одновременно несколько направлений. Отметим ещё раз: в эксперименте по семи направлениям участвуют 53 ИИ-сервиса, большая часть которых демонстрирует вполне обнадёживающие результаты. Подробный анализ будет представлен в наших последующих публикациях.
ОЦЕНКА РАЗМЕРА И ДИНАМИКИ РОССИЙСКОГО РЫНКА ИСКУССТВЕННОГО ИНТЕЛЛЕКТА ДЛЯ ЗДРАВООХРАНЕНИЯ
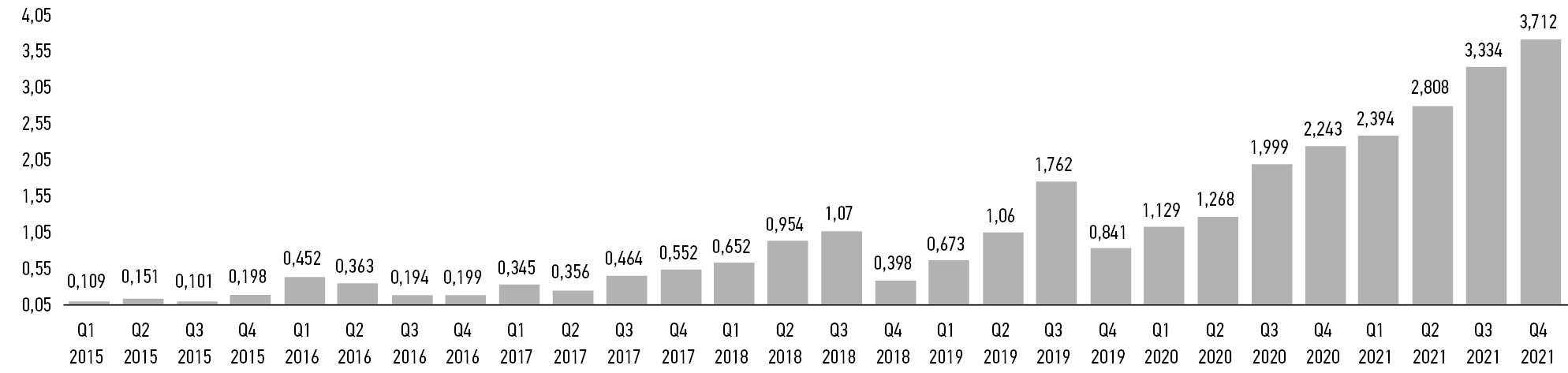
Мы проанализировали публикации, размещённые в средствах массовой информации, об инвестициях в российские компании, предлагающие специализированные ИИ-продукты для здравоохранения, сведения на сайтах соответствующих компаний-разработчиков, а также данные, полученные от ряда государственных институтов, об объёмах государственной поддержки через грантовые и иные инструменты. На основе этой информации мы оценили динамику в продукты ИИ для здравоохранения в России (рис. 3). По итогам 2021 г. суммарные российские инвестиции в ИИ для здравоохранения составили 5,39 млн долларов, или 0,04% от общемирового уровня, при этом в России в 2021 г. объём инвестиций сократился в 3,2 раза по сравнению с 2020 г., в мире ― вырос в 1,8 раза.
Рис. 3. Динамика российских инвестиций в системы искусственного интеллекта для медицины и здравоохранения в 2015–2021 гг. (данные авторов), млн руб.
Структура источников российских инвестиций в ИИ-продукты представлена на рис. 4. Как видно, практически 70% российских инвестиций в разработку ИИ-продуктов осуществляется за счёт государственных источников финансирования, включая институты поддержки, такие как фонды Сколково, Национальной технологической инициативы (НТИ), Фонд содействия инновациям и т.д. Распределение инвестиций по секторам рынка представлено на рис. 5.
Рис. 4. Источники российского инвестирования в системы искусственного интеллекта для медицины и здравоохранения (данные авторов), млн руб.
Рис. 5. Направления инвестирования в системы искусственного интеллекта для медицины и здравоохранения (данные авторов), млн руб.
Примечание. НТИ ― Фонд Национальной технологической инициативы; РФПИ ― Российский фонд прямых инвестиций; ФРИИ ― Фонд развития интернет-инициатив.
Выручка присутствующих на российском рынке компаний системно увеличивается, динамика роста представлена на рис. 6. Несмотря на формальный рост этого показателя, необходимо отметить пока ещё достаточно рискованную структуру выручки с точки зрения зрелости рынка: львиная доля поступлений российских компаний получена за счёт участия в московском эксперименте, а также других грантовых инструментов поддержки, в том числе со стороны отраслевых институтов развития. Данная ситуация свидетельствует о том, что пока российские компании не смогли найти масштабируемые и рыночные инструменты продвижения своей продукции, что может объясняться спецификой регулирования государством в целом рынков здравоохранения.
Рис. 6. Динамика выручки российских разработчиков системы искусственного интеллекта для медицины и здравоохранения в 2017–2020 гг. (данные авторов), млн руб.
Основываясь на выявленных данных по выручке российских ИИ-стартапов, мы пришли к оценке размера рынка ИИ для здравоохранения по итогам 2021 г. порядка 700 млн руб., или 0,11% общемирового показателя.
Общая капитализация российских компаний-разработчиков на основе среднерыночного мультипликатора и выручки оценивается нами в 200 млн долларов, этот же показатель для всего мира составляет 49 млрд долларов. Таким образом, наша доля в этом показателе ― 0,4%.
Всего на конец 2021 г. более чем в 20 субъектах Российской Федерации были запущены разнообразные проекты внедрения ИИ-продуктов, в том числе реализованные в виде пилотных проектов или научно-практических экспериментов. Ключевым драйвером роста интереса к ИИ в России стала пандемия COVID-19.
ЗАКЛЮЧЕНИЕ
Представленные данные наглядно показывают, что России необходимо предпринимать дополнительные системные усилия по развитию исследований, разработок и внедрению продуктов на основе ИИ-технологий для здравоохранения. Эта сфера представляет собой один из крупнейших и жизненно важных сегментов цифровой экономики, и отставание здесь может оказаться крайне критичным.
Одной из проблем низких результатов, как минимум с точки зрения рынка, является отсутствие масштабируемых продаж и массовых внедрений в практическом здравоохранении у российских компаний.
Мы предполагаем, что у этого явления могут быть три ключевые причины. Во-первых, отсутствие целевого государственного финансирования внедрения ИИ-систем в медицинских организациях и органах управления здравоохранением в части инновационных ИИ-продуктов и сервисов. В действующих федеральных программах и национальных проектах, включая проект создания единого цифрового контура в сфере здравоохранения, нет ни мероприятий, ни средств на внедрение ИИ. В номенклатуре медицинских услуг отсутствуют услуги, которые можно было бы оказывать с применением ИИ-технологий, что создаёт барьер для роста покупок соответствующих продуктов как минимум в государственном здравоохранении. Во-вторых, недостаточно выраженная ценность у предлагаемых решений. Многие компании сосредоточены на совершенствовании своих продуктов, повышении точности работы алгоритмов машинного обучения, но при этом недостаточно много внимания уделяют научным исследованиям и предоставлению убедительных доказательств эффективности и ценности своих решений для отечественного здравоохранения. И третье ― недоверие к продуктам. Немногие из российских разработчиков уделяют должное внимание проведению правильно организованных клинических исследований и испытаний своих продуктов. В настоящее время на ИИ-системы выдано 11 регистрационных удостоверений Росздравнадзора семи компаниям-разработчикам. Но в медицине вопросы доверия к новым технологиям являются ключевыми: здесь в первую очередь при принятии решения о применении чего-то нового вспоминают принцип «не навреди».
Представляется, что решение проблем с источниками оплаты за внедрение и использование ИИ-продуктов в практическом здравоохранении, проведение дополнительных научных исследований относительно доверия к качеству ИИ-продуктов и усиление их ценности для потребителей ― те главные стратегические задачи, которые должны быть решены в Российской Федерации для обеспечения роста исследований, разработок и реального применения технологий ИИ в медицинских организациях нашей страны.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Поисково-аналитическая работа не имела спонсорской поддержки.
Конфликт интересов. Авторы заявляют об отсутствии явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов. Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией). Наибольший вклад распределён следующим образом: А.В. Гусев ― идея работы, обзор продуктов и проектов, разделы о нормативном регулировании и данные по рыночным показателям; А.В. Владзимирский ― общая редакция всей статьи, заключение; Д.Е. Шарова ― раздел о нормативном и техническом регулировании; К.М. Арзамасов ― описание московского эксперимента; А.Е. Храмов ― история и текущие данные о научных исследованиях в сфере искусственного интеллекта в здравоохранении.
Благодарности. Авторы выражают благодарность Федеральной службе по надзору в сфере здравоохранения и лично заместителю начальника Управления организации государственного контроля и регистрации медицинских изделий М.М. Сухановой за оказанную помощь в написании настоящей статьи. Выражаем также благодарность Президентской программе поддержки ведущих научных школ Российской Федерации (грант НШ-589.2022.1.2).
ADDITIONAL INFORMATION
Funding source. The article has no sponsorship.
Competing interests. The authors declare that there is no conflict of interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. A.V. Gusev ― the idea, the overview of products and projects, sections on regulation and data on market performance; A.V. Vladzymyrskyy ― the general version of the entire article, the conclusion; D.E. Sharova ― a section on policy management and technical regulation; K.M. Arzamasov ― the description of the Moscow experiment; A.E. Khramov ― the history and current data on research in the field of AI in healthcare.
Acknowledgments. The authors express their gratitude to the Federal Service for Surveillance in Healthcare and personally to the Deputy Head of the Department of the State Control and Registration of Medical Devices M.M. Sukhanova for their assistance in writing this article. We also express our gratitude to the Presidential Program for Support of Leading Scientific Schools of the Russian Federation (grant NSh-589.2022.1.2).
1 Companies in the artificial intelligence in healthcare market are introducing AI-powered surgical robots to improve precision as per the business research company’s artificial intelligence in healthcare global market report 2022 [Internet]. Режим доступа: https://www.globenewswire.com/news-release/2022/03/30/2413072/0/en/Companies-In-The-Artificial-Intelligence-In-Healthcare-Market-Are-Introducing-AI-Powered-Surgical-Robots-To-Improve-Precision-As-Per-The-Business-Research-Company-s-Artificial-Inte.html. Дата обращения: 15.03.2022.
2 Частная американская компания с платформой бизнес-аналитики и глобальной базой данных, которая предоставляет рыночную информацию о частных компаниях и деятельности инвесторов.
3 State of AI 2021 Report [Internet]. Режим доступа: https://www.cbinsights.com/research/report/ai-trends-2021/. Дата обращения: 15.03.2022.
4 Указ Президента РФ от 10.10.2019 № 490 «О развитии искусственного интеллекта в Российской Федерации» (вместе с «Национальной стратегией развития искусственного интеллекта на период до 2030 года»). Режим доступа: http://www.consultant.ru/document/cons_doc_LAW_335184/. Дата обращения: 15.03.2022.
5 Указ Президента РФ от 07.05.2018 № 204 «О национальных целях и стратегических задачах развития Российской Федерации на период до 2024 года». Режим доступа: http://publication.pravo.gov.ru/Document/View/0001201805070038. Дата обращения: 15.03.2022.
6 Распоряжение Правительства РФ от 19.08.2020 № 2129-р «Об утверждении Концепции развития регулирования отношений в сфере технологий искусственного интеллекта и робототехники на период до 2024 года». Режим доступа: https://www.garant.ru/products/ipo/prime/doc/74460628/. Дата обращения: 15.03.2022.
7 Федеральный закон от 31.01.2016 № 4-ФЗ «О ратификации Соглашения о единых принципах и правилах обращения медицинских изделий (изделий медицинского назначения и медицинской техники) в рамках Евразийского экономического союза». Режим доступа: https://ipbd.ru/doc/0001201601310008/. Дата обращения: 15.03.2022.
8 Федеральный закон от 21.11.2011 № 323-ФЗ «Об основах охраны здоровья граждан в Российской Федерации» (последняя редакция). Режим доступа: http://www.consultant.ru/document/cons_doc_LAW_121895/. Дата обращения: 15.03.2022.
9 Режим доступа: https://base.garant.ru/70291692/. Дата обращения: 15.03.2022.
10 Режим доступа: http://www.consultant.ru/document/cons_doc_LAW_368970/92d969e26a4326c5d02fa79b8f9cf4994ee5633b/. Дата обращения: 15.03.2022.
11 Режим доступа: https://base.garant.ru/71626748/. Дата обращения: 15.03.2022.
12 Режим доступа: https://docs.cntd.ru/document/608935477. Дата обращения: 15.03.2022.
13 Режим доступа: https://docs.cntd.ru/document/902353334. Дата обращения: 15.03.2022.
14 Режим доступа: http://publication.pravo.gov.ru/Document/View/0001202008100015. Дата обращения: 15.03.2022.
15 Коллегия Евразийской экономической комиссии. Рекомендация от 12.11.2018 № 25 «О Критериях отнесения продукции к медицинским изделиям в рамках Евразийского экономического союза (с изменениями на 29.06.2021)». Режим доступа: https://docs.cntd.ru/document/551663485. Дата обращения: 15.03.2022.
16 Приказ Министерства промышленности и торговли Российской Федерации, Федерального агентства по техническому регулированию и метрологии от 25.07.2019 № 1732 «О создании технического комитета по стандартизации «Искусственный интеллект»». Режим доступа: https://docs.cntd.ru/document/560916332. Дата обращения: 15.03.2022.
17 Приказ Министерства промышленности и торговли Российской Федерации, Федерального агентства по техническому регулированию и метрологии от 31.12.2019 № 3471 «О внесении изменений в приказ Федерального агентства по техническому регулированию и метрологии от 25.07.2019 № 1732 «О создании технического комитета по стандартизации «Искусственный интеллект»». Режим доступа: https://docs.cntd.ru/document/564243465. Дата обращения: 15.03.2022.
18 Постановление Правительства Российской Федерации от 24.11.2020 № 1906 «О внесении изменений в Правила государственной регистрации медицинских изделий». Режим доступа: http://publication.pravo.gov.ru/Document/View/0001202011270010. Дата обращения: 15.03.2022.
19 Указ Президента РФ от 10.10.2019 № 490 «О развитии искусственного интеллекта в Российской Федерации» (вместе с «Национальной стратегией развития искусственного интеллекта на период до 2030 года»). Режим доступа: http://www.consultant.ru/document/cons_doc_LAW_335184/. Дата обращения: 15.03.2022.
20 SberPress [интернет]. На ICML Сбер представил свои разработки на основе искусственного интеллекта в медицине. Режим доступа: https://press.sber.ru/publications/na-icml-sber-predstavil-svoi-razrabotki-na-osnove-iskusstvennogo-intellekta-v-meditsine. Дата обращения: 15.03.2022.
Об авторах
Александр Владимирович Гусев
К-Скай; Центральный научно-исследовательский институт организации и информатизации здравоохранения
Автор, ответственный за переписку.
Email: agusev@webiomed.ai
ORCID iD: 0000-0002-7380-8460
SPIN-код: 9160-7024
Scopus Author ID: 57222273391
ResearcherId: AAD-2073-2019
к.т.н.
Россия, Петрозаводск; МоскваАнтон Вячеславович Владзимирский
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: a.vladzimirsky@npcmr.ru
ORCID iD: 0000-0002-2990-7736
SPIN-код: 3602-7120
Scopus Author ID: 8944262100
ResearcherId: D-1447-2017
Россия, Москва
Дарья Евгеньевна Шарова
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: d.sharova@npcmr.ru
ORCID iD: 0000-0001-5792-3912
SPIN-код: 1811-7595
Россия, Москва
Кирилл Михайлович Арзамасов
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: k.arzamasov@npcmr.ru
ORCID iD: 0000-0001-7786-0349
SPIN-код: 3160-8062
Россия, Москва
Александр Евгеньевич Храмов
Университет Иннополис; Балтийский федеральный университет имени Иммануила Канта
Email: a.hramov@innopolis.ru
ORCID iD: 0000-0003-2787-2530
SPIN-код: 7357-7556
Scopus Author ID: 34834
Россия, Казань; Калининград
Список литературы
- Deep medicine: how artificial intelligence can make healthcare human again by eric topol. New York: Basic Books, 2019. 341 p.
- Roberts M., Driggs D., Thorpe M., et al. Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans // Nat Mach Intel. 2021. Vol. 3, N 3. P. 199–217. doi: 10.1038/s42256-021-00307-0
- Wynants L., Van Calster B., Collins G.S., et al. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal // BMJ. 2020. Vol. 369. P. m1328. doi: 10.1136/bmj.m1328
- Гусев А.В., Морозов С.П., Кутичев В.А., Новицкий Р.Э. Нормативно-правовое регулирование программного обеспечения для здравоохранения, созданного с применением технологий искусственного интеллекта, в Российской Федерации // Медицинские технологии. Оценка и выбор. 2021. № 1. С. 36–45. doi: 10.17116/medtech20214301136
- Шарова Д.Е., Зинченко В.В., Ахмад Е.С., и др. К вопросу об этических аспектах внедрения систем искусственного интеллекта в здравоохранении // Digital Diagnostics. 2021. Т. 2, № 3. С. 356–368. doi: 10.17816/DD77446
- Морозов С.П., Зинченко В.В., Хоружая А.Н., и др. Стандартизация искусственного интеллекта в здравоохранении: Россия выходит в лидеры // Врач и информационные технологии. 2021. № 2. С. 12–19. doi: 10.25881/18110193_2021_2_12
- Морозов С.П., Владзимирский А.В., Шарова Д.Е., и др. Первые национальные стандарты Российской Федерации на системы искусственного интеллекта в медицине // Менеджмент качества в медицине. 2022. № 1. С. 58–62.
- Комарь П.А., Дмитриев В.С., Ледяева А.М., и др. Рейтинг стартапов искусственного интеллекта: перспективы для здравоохранения России // Российский журнал телемедицины и электронного здравоохранения. 2021. Т. 7, № 3 С. 32–41. doi: 10.29188/2712-9217-2021-7-3-32-41
- Корсаков С.Н. Начертание нового способа исследования при помощи машин, сравнивающих идеи / пер. с франц.; под ред. А.С. Михайлова. Москва: МИФИ, 2009. 44 c.
- Гаврилова Т.А., Хорошевский В.Ф. Базы знаний интеллектуальных систем: учебное пособие. Санкт-Петербург: Питер, 2000. 384 с.
- Danilov V.V., Proutski A., Karpovsky A., et al. Indirect supervision applied to COVID-19 and pneumonia classification // Informatics Medicine Unlocked. 2022. Vol. 28. P. 100835. doi: 10.1016/j.imu.2021.100835
- Mohammadi R., Salehi M., Ghaffari H., et al. Transfer learning-based automatic detection of coronavirus disease 2019 (COVID-19) from chest X-ray images // J Biomed Phys Eng. 2020. Vol. 10, N 5. Р. 559–568. doi: 10.31661/jbpe.v0i0.2008-1153
- Нероев В.В., Брагин А.А., Зайцева О.В. Разработка прототипа сервиса для диагностики диабетической ретинопатии по снимкам глазного дна с использованием методов искусственного интеллекта // Национальное здравоохранение. 2021. Т. 2, № 2. С. 64–72. doi: 10.47093/2713-069X.2021.2.2.64-7
- Невзорова В.А., Бродская Т.А., Шахгельдян К.И., и др. Методы машинного обучения в прогнозировании рисков 5-летней смертности (по данным исследования ЭССЕ-РФ в Приморском крае) // Кардиоваскулярная терапия и профилактика. 2022. Т. 21, № 1. С. 2908. doi: 10.15829/1728-8800-2022-2908
- Гиляревский С.Р., Гаврилов Д.В., Гусев А.В. Результаты ретроспективного анализа записей электронных амбулаторных медицинских карт пациентов с хронической сердечной недостаточностью: первый российский опыт // Российский кардиологический журнал. 2021. Т. 26, № 5. С. 4502. doi: 10.15829/1560-4071-2021-4502
- Karpov O.E., Grubov V.V., Maksimenko V.A., et al. Noise amplification precedes extreme epileptic events on human EEG // Physical Review. 2021. Vol. 103. Р. 022310. doi: 10.1103/PhysRevE.103.022310
- Кучин А.С., Грубов В.В., Максименко В.А., Утяшев Н.П. Автоматизированное рабочее место врача-эпилептолога с возможностью автоматического поиска приступов эпилепсии // Врач и информационные технологии. 2021. № 3. Р. 62–73 doi: 1025881/18110193_2021_3_62
- Kuc A., Korchagin S., Maksimenko V.A., et al. Combining statistical analysis and machine learning for eeg scalp topograms classification // Frontiers Systems Neuroscience. 2021. Vol. 15. Р. 716897. doi: 10.3389/fnsys.2021.716897
- Hramov A.E., Maksimenko V.A., Pisarchik A.N. Physical principles of brain-computer interfaces and their applications for rehabilitation, robotics and control of human brain states // Physics Reports. 2021. Vol. 918. Р. 1–133. doi: 10.1016/j.physrep.2021.03.002
- Морозов С.П., Владзимирский А.В., Шулькин И.М., и др. Исследование целесообразности применения технологий искусственного интеллекта в лучевой диагностике // Врач и информационные технологии. 2022. № 1. С. 12–29.
Дополнительные файлы