Антропоморфные фантомы молочной железы для лучевой диагностики: научный обзор
- Авторы: Васильев Ю.А.1, Омелянская О.В.1, Насибуллина А.А.1, Леонов Д.В.1, Булгакова Ю.В.1, Ахмедзянова Д.А.1, Шумская Ю.Ф.1, Решетников Р.В.1
-
Учреждения:
- Научно-практический клинический центр диагностики и телемедицинских технологий
- Выпуск: Том 4, № 4 (2023)
- Страницы: 569-592
- Раздел: Обзоры
- Статья получена: 14.11.2023
- Статья одобрена: 27.11.2023
- Статья опубликована: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/623341
- DOI: https://doi.org/10.17816/DD623341
- ID: 623341
Цитировать
Аннотация
Фантомы молочной железы применяются для разработки, валидации и усовершенствования методов лучевой диагностики. В визуализации молочной железы антропоморфные модели используются для валидации, оценки и оптимизации новых методов диагностики заболеваний молочной железы, а также для контроля качества диагностических систем, совершенствования клинических протоколов и алгоритмов реконструкции изображений. Ключевым требованием к фантомам для решения этих задач является реалистичная имитация органа.
В обзоре описаны существующие на настоящий момент варианты фантомов молочной железы для лучевой диагностики и процесса их создания.
Поиск литературы, соответствующей теме обзора, производился в базах данных PubMed, eLibrary, а также в поисковой системе Google Scholar. Всего в обзор включено 72 статьи и 13 тезисов материалов конференций.
Все виды фантомов молочной железы можно разделить на два вида: вычислительные и физические. Вычислительные, в свою очередь, подразделяются на группы в зависимости от типа первичных данных: на основе математических моделей, из образцов тканей, с использований изображений медицинской визуализации молочной железы пациентки. Физические фантомы классифицируются в зависимости от способа изготовления: литья, 3D-печати или послойного формирования с использованием контрастных веществ. Основными преимуществами вычислительных фантомов являются универсальность, эффективность, точность и безопасность, а также возможность генерировать большие объёмы виртуальных данных. Физические фантомы позволяют получать наиболее реалистичные диагностические изображения без участия пациентов и проводить неограниченное число лучевых исследований.
Ключевые слова
Полный текст
ВВЕДЕНИЕ
Фантомы человека — ключевой инструмент для решения многих задач в области медицинской визуализации для ряда модальностей: ультразвукового исследования [1], магнитно-резонансной (МРТ) [2] и компьютерной (КТ) томографий [3] и других. Фантомы молочной железы (МЖ) являются важным элементом оценки систем рентгеновской визуализации, служа заменой человеческому телу в тех случаях, когда облучение пациента нецелесообразно или не нужно. Цель создания фантомов МЖ для рентгенодиагностики — разработка, оптимизация и контроль качества существующих и разрабатываемых систем визуализации, таких как широкопольная цифровая маммография (ММГ), цифровой томосинтез и КТ.
Антропоморфные фантомы МЖ, предназначенные для применения в рентгенодиагностике, представляют собой как вычислительные (математические), так и физические модели МЖ. Математические модели представляют собой описание структур органа с использованием формул и расчётов. Одним из ограничений этого подхода является невозможность охватить всё многообразие анатомических вариаций строения МЖ и обеспечить её анатомическую и рентгенологическую достоверность. Для преодоления этих ограничений на основе сегментированных наборов данных, полученных при КТ МЖ, создаются антропоморфные фантомы с естественным распределением различных типов тканей, что гарантирует высокую степень реалистичности. Попытки создания таких антропоморфных вычислительных фантомов предпринимались с 1960 года [4], но лишь недавно, благодаря появлению технологий трёхмерной (3D) печати, стали появляться достоверные антропоморфные физические фантомы. Основные методы создания физических антропоморфных моделей МЖ — литьё, 3D-печать и подходы с использованием бумаги. Среди вышеперечисленного именно технологии 3D-печати дают прекрасную возможность создавать реалистичные модели за счёт использования материалов, рентгеновские характеристики которых схожи с характеристиками тканей МЖ.
В связи с расширением клинического применения современных квази-3D и 3D-систем визуализации, таких как томосинтез и КТ, существует большая потребность в физических фантомах с реалистичной анатомией пациента для полного отображения и оценки 3D-поведения таких систем. Кроме того, принимая во внимание активное и успешное внедрение систем искусственного интеллекта в анализ изображений ММГ [5], применение фантомов позволит достичь стандартизации рентгенологических исследований, что поможет улучшить алгоритмы искусственного интеллекта.
Методология поиска
Поиск литературы, соответствующей теме обзора, производился в базе данных PubMed и eLibrary. Использованные поисковые запросы были подобраны так, чтобы наилучшим образом отобразить ключевые слова и предметные рубрики. Поиск в базе данных PubMed осуществлялся по запросу: «anthropomorphic AND breast* AND (phantom* OR phantoms, imaging [mh] OR (phantoms AND imaging) OR model*) AND (diagnostic x-ray OR radiography OR mammography OR tomography)». Поиск в базе данных eLibrary осуществлялся по ключевым словам: «фантом», «молочная железа». В поисковой системе Google Scholar был также произведён поиск материалов конференций.
РЕЗУЛЬТАТЫ
В результате поиска было выявлено 335 статей, однако 263 статьи были признаны не соответствующими необходимым для данного обзора критериям. В обзор было включено 72 статьи, а также 13 тезисов материалов конференций.
Анатомия молочной железы
Для точного моделирования МЖ при рентгеновской визуализации необходимы детальные знания о её анатомических и рентгенологических особенностях. МЖ — видоизменённая кожная потовая железа, которая состоит из железистой ткани тубулоальвеолярного типа, соединительной ткани и других элементов: жировой ткани, кровеносных и лимфатических сосудов, а также нервных волокон. Наибольший объём занимают железистая и жировая ткани. Каждая МЖ располагается на фасции, покрывающей большую грудную мышцу на уровне III–VI ребра между передней подмышечной и окологрудинной линиями соответствующей стороны. МЖ также окружена передним и задним листком поверхностной фасции груди, вместе образующими капсулу для железы. Поверхностная фасция прикрепляется к ключице и образует поддерживающую связку. От заднего листка вглубь МЖ и от переднего листка по направлению к коже отходят соединительнотканные перегородки — связки Купера.
МЖ состоит из 15–20 долек, расположенных радиально по отношению к соску, окружённых рыхлой соединительной и жировой тканью. В каждой дольке есть свой молочный проток. В субареолярной области молочные протоки расширяются, образуя молочные синусы (ампулы). В них протоки нескольких долей сливаются в более крупные, которые выходят в сосок самостоятельно, образуя отдельные отверстия на его поверхности. Диаметр протоков до ампулы составляет около 1 мм, в то время как диаметр основных протоков варьирует от 2 до 4,5 мм. Общая длина протоков варьирует от 2 до 4,5 см. Долька диаметром 1–2 мм состоит из протоков и альвеол, которые заканчиваются одним общим терминальным протоком. Эта структура называется «терминальная долько-протоковая единица».
Чётко определённой строгой нормы рентгенологической картины МЖ не существует ввиду разнообразного соотношения железистой и жировой тканей у разных женщин. Соответственно, разработаны несколько классификаций маммографических видов плотности: Вульфа (Wolf), BI-RADS, Tabar. Указанные классификации представлены в табл. 1. Соответственно, целью моделирования фантома МЖ являются те или иные категории выбранной исследователями классификации.
Таблица 1. Классификации типов строения молочных желёз
Тип | Характеристика |
Классификация Wolf | |
N1 | МЖ состоит преимущественно из жировой ткани (N = норма); соответствует низкому риску развития рака МЖ |
P1 | данный тип включает наличие жировой ткани и линейных плотностей (расширенные протоки), занимающих не более 25% МЖ; соответствует низкому риску развития рака МЖ |
P2 | линейные плотности (расширенные протоки), занимающие более 25% МЖ. Они преимущественно локализуются в верхнем наружном квадранте, но могут быть распределены по всей груди (P = видимые протоки); соответствует высокому риску развития рака МЖ |
Dy | плотная МЖ (Dy = дисплазия); соответствует наибольшему риску развития рака МЖ |
Qdy | квази-дисплазия; к этой группе относятся молодые женщины, у которых плотная структура МЖ имеет губчатую структуру за счёт жировой инфильтрации |
Классификация BI-RADS | |
a | МЖ с преимущественным жировым компонентом, наличие фиброгландулярной ткани менее 25% площади маммограмм |
b | есть очаги фиброгландулярной ткани, занимающие от 25 до 50% площади на маммограммах |
c | МЖ с гетерогенными плотностями (фиброгландулярная ткань), занимающими от 51 до 75 % площади маммограммы |
d | очень плотные МЖ, процент фиброгландулярной ткани — более 75% площади маммограммы |
Классификация Tabar | |
I | сбалансированное соотношение тканей МЖ с небольшим преобладанием фиброзной ткани |
II | преобладание жировой ткани |
III | преобладание жировой ткани с наличием ретроареолярно фиброзной ткани |
IV | ткань МЖ представлена преимущественно узловыми уплотнениями |
V | преобладание фиброзной ткани |
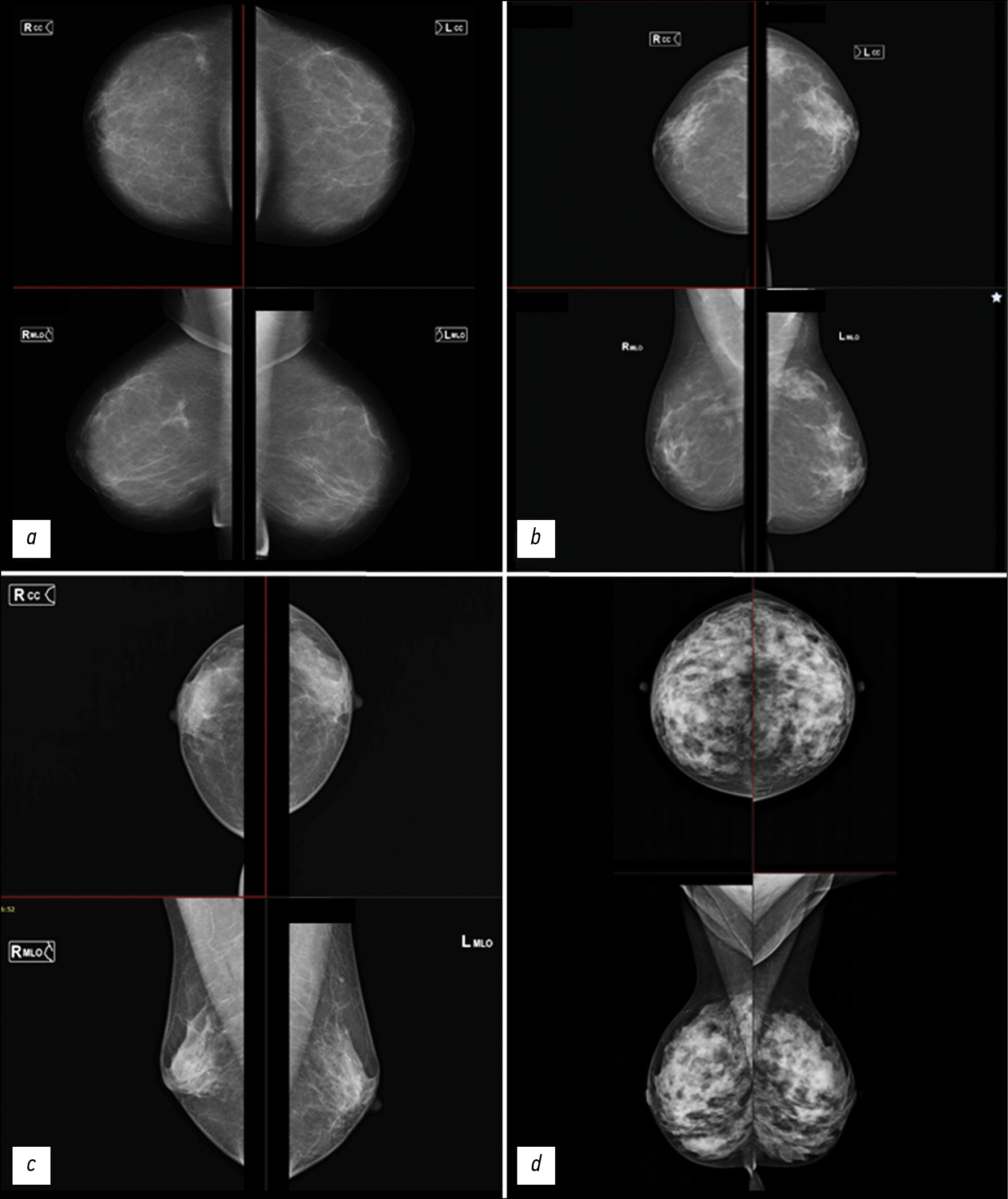
Классификация BI-RADS является наиболее широко применяемой для описания рентгеновской плотности МЖ в клинической практике. Типы структур ткани МЖ по типам представлены на рис. 1.
Рис. 1. Типы плотности ткани молочной железы согласно классификации BI-RADS. Для каждого изображения верхняя часть — краниокаудальная проекция, нижняя часть — медиолатеральная проекция.
Для моделирования анатомических структур МЖ могут использоваться как математические модели, так и диагностические изображения пациенток. На основе этих данных при изготовлении физических антропоморфных фантомов применяют три основных подхода:
- использование методов литья;
- печать различных структур по отдельности с последующей сборкой полного фантома;
- печать модели целиком.
Вычислительные трёхмерные антропоморфные модели молочных желёз
Антропоморфные вычислительные модели МЖ обладают анатомически достоверным объёмным распределением величин коэффициентов поглощения рентгеновского излучения различными типами тканей МЖ. Можно выделить три подхода к их созданию:
- Подход, основанный на математическом моделировании.
Фантомы на основе математических моделей разработаны таким образом, чтобы соответствовать определённым физическим или статистическим свойствам органа человека, таким как рентгеновская плотность или статистические закономерности распределения определённых характеристик [6]. Несколько исследовательских групп разработали надёжные виртуальные фантомы МЖ на основе моделей для использования в проекционной и томографической визуализации [7–10]. Схема создания такого типа фантомов представлена на рис. 2. - Подход, основанный на анализе образцов ткани.
Эти фантомы воспроизводят мелкомасштабную структуру ткани МЖ, наблюдаемую в образцах патологии или микротомографии сверхвысокого разрешения [11, 12]. - Подход, основанный на обработке томограмм пациентов.
Рис. 2. Схема строения вычислительного фантома молочной железы.
Такие модели создаются на основе двухмерных (ММГ) или трёхмерных (КТ или МРТ) снимков [13, 14].
Математические модели молочной железы
Данный тип моделей основан на моделировании элементов строения МЖ с помощью математических методов. Такие модели комплексно отображают форму органа, протоковую систему, связки Купера, большую грудную мышцу, сосуды, кожу, ММГ-рисунок и патологию МЖ. Для создания трёхмерной анатомически реалистичной модели МЖ, K. Bliznakova и соавт. [15], а также P.R. Bakic и соавт. [16–18], использовали комбинацию методов Constructive Solid Geometry и воксельных техник.
Модель молочной железы Пенсильванского Университета
В 2002 г. исследовательская группа из Пенсильванского университета разработала первый антропоморфный вычислительный фантом МЖ, реализованный путём моделирования двух эллипсоидных областей крупномасштабных тканевых элементов: преимущественно жировой ткани и преимущественно фиброгландулярной ткани [16, 17]. Эти области математически моделируются реалистично распределёнными среднемасштабными элементами фантома: оболочками, дольками и смоделированными молочными протоками. Модель сети протоков реализуется с использованием матрицы разветвления, описывающей характер дихотомизации древовидных структур [18]. Достоверность модели млечных протоков определяли в сравнении с данными дуктографии. Разработана также модель сжатия, достоверно воспроизводящая деформацию МЖ, необходимая для применения модели Пенсильванского Университета в двухмерной ММГ и томосинтезе МЖ. Дальнейшая работа над этим фантомом заключалась в добавлении мелких структур и реализации алгоритма для быстрой генерации антропоморфных фантомов высокого разрешения с размером вокселя в диапазоне 25–1000 мкм3 [19]. Таким образом, была создана усовершенствованная версия этого цифрового фантома [12].
Предложенные алгоритмы основаны на упрощённом предположении, что каждый воксель фантома содержит один тип ткани. Такое упрощение ведёт к появлению артефактов на проекционных изображениях вблизи границ между областями из разных материалов, особенно на границе кожа–воздух. Для преодоления этих ограничений было предложено несколько методов. Один из них — моделирование вокселей меньшего размера. Однако уменьшение размера вокселей увеличивает время генерации фантома и повышает требования к характеристикам электронно-вычислительной машины, на которой моделируется фантом. Другой метод заключается в учёте частичного объёма различных тканей в вокселе [20, 21]. Коэффициент линейного ослабления рентгеновского излучения в каждом вокселе рассчитывается путём комбинирования коэффициентов ослабления, пропорциональных субобъёмам вокселя, занимаемым различными тканями.
Модель молочной железы Университета Патраса
Параллельно исследовательская группа из Университета Патраса разработала антропоморфную модель МЖ, которая представляет собой сложную совокупность анатомической формы, системы протоков, связок Купера, рентгенологической текстуры ткани и узлов [15]. 3D-текстура моделирует наличие жировой, фиброзной и соединительной, а также других типов тканей, которые не моделируются в явном виде. Для получения реалистичной 3D ММГ-текстуры применяется алгоритм текстурирования, основанный на случайном блуждании (random walk) [10]. Связки Купера моделируются как набор тонких эллипсоидных оболочек, возникающих в произвольно выбранных позициях модели. Жировые компартменты моделируются объёмом, заключённым в связках Купера. Грудная мышца аппроксимируется в виде объекта конической формы, а узлы моделируются объектами круглой, овоидной, вытянутой или неправильной формы.
Фантом молочной железы United States Food and Drug Administration
На основе описанных выше разработок исследовательская группа из Управления по контролю качества пищевых продуктов и лекарственных средств Соединённых Штатов Америки представила усовершенствованный мультимодальный математический фантом МЖ с открытым исходным кодом для использования научным сообществом [21]. МЖ генерируются на основе аналитических формул с последующей воксельной дискретизацией. При этом каждый воксель имеет произвольный размер и состоит из одного типа ткани. К передней части поверхности добавляется сосок и слой кожи толщиной в 1 мм, а к задней — слой мышц, поддерживающих грудь. Во внутренней поверхности на основе сегментации Вороного определяются железистые отделы. Для каждого сегментированного отсека железы с помощью алгоритма случайного ветвления выращивается дерево протоков, начиная от соска. В терминальных ветвях каждого «древа» протоков добавляются терминальные дольковые протоки. Изначально внутренняя часть фантома задаётся как чисто железистая ткань. Для создания подкожного и перилобулярного жировых слоёв, а также некоторых жировых структур, внутри железистых областей вставляются случайные жировые дольки. Каждая жировая долька окружена связочной структурой [22]. На финальном этапе генерации фантома вводятся дополнительные структуры, такие как кровеносные сосуды, грудные мышцы и связки.
Модель молочной железы OPTIMAM
Параллельно с разработкой Управления по контролю качества пищевых продуктов и лекарственных средств Соединённых Штатов Америки, P. Elangovan и соавт. [9] представили новый метод генерации квазиреалистичных воксельных фантомов, который позволяет имитировать сжатую МЖ, используемую в ММГ и томосинтезе. Антропоморфные модели МЖ создаются биомиметическим подходом, при котором из плоскостей изображений реальных МЖ, полученных при томосинтезе, извлекаются их особенности и структуры. Они используются для синтеза трёхмерной структуры МЖ. Процесс моделирования начинается с создания пустой заготовки МЖ высокого разрешения, окружённой слоем кожи. Эта заготовка заполняется вокселями, соответствующими различным тканям на основе структур, извлечённых из изображений томосинтеза. Общую трёхмерную форму получают из данных томосинтеза с помощью пороговой обработки изображений. Затем к кожным поверхностям контура МЖ добавляется слой «кожи» толщиной 1,5 мм. Внутренние воксели маркируются как жировая или железистая ткань.
Далее создаётся база данных произвольно выбранных фрагментов железистой ткани, используемая для изготовления 3D-матрицы железистой ткани, которая помещается в жировую ткань МЖ. Кровеносные сосуды и связки Купера моделируются в виде расширенного трёхмерного каркаса линейных структур, которые присутствуют на изображениях томосинтеза пациентки: 3–4 мм в диаметре для сети кровеносных сосудов и до 1–2 мм в диаметре для сети связок Купера.
Вычислительные модели молочной железы на основе медицинских данных
Попытки создания более реалистичного распределения тканей в модели МЖ были предприняты несколькими авторами [20, 23, 24]. Три воксельные модели МЖ были созданы на основе сегментированных КТ-данных высокого разрешения трёх МЖ, зафиксированных в сжатом состоянии (взятых у трёх разных пожилых женщин) [25]. Эти сегментированные модели МЖ позволили более реалистично представить железистую ткань и рассчитать методом Монте-Карло среднюю дозу облучения МЖ при ММГ, а также смоделировать различные техники визуализации.
Появление специализированной системы КТ МЖ позволило продвинуться в создании вычислительных антропоморфных фантомов МЖ. Впервые такой фантом был разработан в 2009 году C.M. Li и соавт. [14]. Ключевым этапом являлось создание методики, с помощью которой можно было обработать срезы КТ МЖ и дифференцировать ткани МЖ. Полученное смоделированное ММГ-изображение фантома было сходно с достоверным ММГ-изображением тканей МЖ. Данная методика имела некоторые ограничения, так как не позволяла надёжно классифицировать мелкие структуры (связки Купера), требующие более высокого разрешения. Чтобы решить эту проблему, авторы предложили выражать данные структуры математически и в дальнейшем включать их в расчётный объём МЖ.
Несмотря на то, что подход C.M. Li и соавт. гарантирует высокую степень реалистичности, он представляет собой только одну модель МЖ и не позволяет охватить широкие анатомические вариации. Для решения этой проблемы C.M. Hsu и соавт. [26, 27] разработали вычислительную методику, позволяющую создать большое количество компьютерных антропоморфных фантомов МЖ [14]. Эта методика основана на морфинге (визуальном эффекте, создающем впечатление плавной трансформации одного объекта в другой) и деформации (значительном искажении форм при цифровой обработке изображения) и демонстрирует способность генерировать реалистичные с точки зрения рентгенологов антропоморфные фантомы МЖ.
Для ускорения разработки моделей МЖ на основе данных КТ необходима точная анатомическая характеристика МЖ с помощью КТ. В работе S.Y. Huang и соавт. [28] были предложены и подробно описаны анатомические признаки МЖ: форма, диаметр и длина МЖ, доля железистой ткани в трёх областях МЖ в зависимости от возраста пациентки и размера чашки бюстгальтера. Работа была выполнена на самом большом из имеющихся на тот момент наборе изображений (219 шт.), полученных с помощью КТ МЖ. Кроме того, на их основе исследовательская группа из Университета Дьюка изготовила 224 виртуальных фантома МЖ [29, 30]. Авторы также разработали приложение для моделирования различных состояний сжатия МЖ, что позволило использовать фантомы для мультимодальной визуализации.
A. Sarno и соавт. разработали 88 вычислительных фантомов МЖ с реалистичным распределением железистой ткани для оценки распределения дозы в МЖ и данных визуализации. Эти модели способствуют созданию усовершенствованного фантома, позволяющего более точно рассчитывать средний показатель лучевой нагрузки на железу при рентгенологических методах исследования [31], а также для оптимизации томосинтеза с помощью виртуальных клинических испытаний.
Таким образом, основное преимущество вычислительных фантомов заключается в их способности потенциально генерировать большие объёмы данных [21], но при этом данные фантомы являются виртуальными, а не физическими. Качество исследования in silico зависит не только от характеристик виртуального фантома, но и от точности моделируемой системы визуализации в представлении физической модальности изображения. Например, при томосинтезе или КТ МЖ сканирование виртуального фантома часто требует детального знания геометрии диагностической системы и алгоритма реконструкции, который является собственностью компании-производителя.
Физические антропоморфные фантомы молочной железы
На момент написания обзора технологии 3D-печати — наиболее популярный подход к созданию физических моделей МЖ. Технологии 3D-печати позволяют выбирать между различными методами печати и материалами, сходными с тканями человека по плотности, компонентному составу и рентгенологическим свойствам. В случае МЖ такими тканями являются жировая, железистая, опухолевая и кожа.
Процесс создания антропоморфного физического фантома МЖ состоит из двух этапов:
- создание вычислительной модели МЖ с использованием одного из методов, рассмотренных в предыдущих разделах;
- использование вычислительной модели для изготовления физического фантома МЖ.
Огромные усилия направлены на разработку новых материалов, поскольку существующие не всегда обладают необходимыми рентгенологическими свойствами [32–34]. Обширный обзор материалов, используемых при изготовлении антропоморфных моделей, представлен в статье K. Bliznakova и соавт. [35], а некоторые основные материалы, применяемые для изготовления фантомов МЖ, представлены в табл. 2.
Таблица 2. Основные материалы, используемые при создании фантомов молочной железы для маммографических исследований
Человеческая ткань | Материал | Плотность, г/см³ |
Жировая ткань | Сверхвысокомолекулярный полиэтилен высокой плотности [36] | 0,94 |
Пластик из акрилонитрила с бутадиеном и стиролом [32] | 1,02 | |
Полиэтилен PE-12 [37] | N/A | |
Фотополимер прозрачного цвета Formlabs Clear Resin [38] | 1,18 | |
QuickWater [33] | 1,02 | |
Парафин [32] | 0,93 | |
Железистая ткань | Поливиниловый спирт (PVAL gel) [39] | 1,19 |
Вода [40] | 1,00 | |
Нейлон [32] | 1,11 | |
Flex-полимер [32] | 1,14 | |
TangoBlackPlus [33] | 1,11 | |
VeroClear [33] | 1,18 | |
VeroWhitePlus, TangoPlus Полилактин, легированный с медью и водой [41] | N/A | |
Поливиниловый спирт [42] | N/A | |
Фотополимер Formlabs Grey resin [32] | 1,175 | |
Опухолевая ткань | Полиэтилентерефталат [42] | N/A |
Фантомы молочной железы, изготовленные с использованием технологии литья
Фантом для ММГ CIRS BR3D (CIRS Inc., Норфолк, США) изготавливается в виде D-образных пластин, которые соединяются в единую конструкцию и формируют цельный фантом. Каждая пластина состоит из двух тканевых материалов, имитирующих жировую и железистую ткани в соотношении 50/50 по весу. Два тканеэквивалентных пластика смешиваются и образуют гетерогенную структуру. Обычно изготавливается несколько пластин, которые можно переставлять в разном порядке и легко получать различные, но ограниченные по количеству варианты подлежащей ткани. Одна из пластин содержит набор микрокальцинатов, фиброзной ткани и новообразований. Фантом используется при изучении качества изображения и в исследованиях, связанных с методами томосинтеза и КТ МЖ. Полученные изображения структур на ММГ-снимке, хотя и имеют нечёткие границы, выглядят не столь реалистично, как на снимках пациента. Например, мелкие структуры, такие как связка Купера, не могут быть изготовлены с помощью данной технологии и, следовательно, их не будет на рентгеновских снимках. В недавнем исследовании, проведённом J. Sage и соавт. [43], показано, что текстурированный фон BR3D является высококонтрастным и приводит к появлению артефактов высокой контрастности на всём фантоме. Кроме того, на изображениях томосинтеза хорошо видны контуры кольцевидных структур, в которые помещаются вставки, что влияет на результаты измерений и анализа изображений.
Антропоморфный фантом МЖ, предназначенный как для рентгенологического исследования, так и для МРТ, был разработан M. Freed и соавт. [44], которые смешивали яичный белок с растопленным рафинированным салом с последующим размещением полученной смеси в банке, имеющей форму МЖ. Этот фантом оказался полезным инструментом для количественной оценки качества изображения в двух- и трёхмерных рентгеновских методиках. Однако он не позволяет моделировать анатомические структуры, так как в фантоме они выглядят значительно крупнее, чем у пациента. Кроме того, не существует устоявшейся методики изготовления фантомов с достаточным содержанием железистой ткани.
Пресс-формы МЖ использовались G. Ruvio и соавт. [45] для создания мультимодальных фантомов МЖ, предназначенных для рентгеновской, магнитно-резонансной и ультразвуковой визуализации. Процесс изготовления включал пять этапов с использованием трёх различных пресс-форм МЖ: внешней (повторяющей форму МЖ), кожной и внутренней фиброгландулярной. Внешняя форма МЖ повторяет форму МЖ человека в положении лёжа, полученную на основе данных МРТ пациентов. При изготовлении кожного элемента основным материалом является криогель поливинилового спирта. Жировая структура представлена смесью воды и пчелиного воска, а фиброгландулярный, опухолевый и мышечный компоненты представлены агаром, содержащим смесь жидких (вода, глицерин, бензалкония хлорид) и сухих (агар, SiC, Al2O3) компонентов. Существующее в настоящее время ограничение связано с невозможностью сжатия фантома из-за того, что материал, имитирующий жир, не поддаётся упругой деформации. Кроме того, данный фантом хранится не более одной недели, поскольку подвержен влиянию бактерий и высыханию. Это приводит к недостаточному контрасту между опухолью и фиброгландулярной тканью. Несмотря на сложность работы по изготовлению тканей, имитирующих важные параметры визуализации для трёх модальностей, в ходе исследования было продемонстрировано высокое сходство между эталонными и измеренными свойствами. Данный тип фантомов предназначен для использования в мультимодальной перекрёстной калибровке и при обучении, когда нет живых пациентов или трупного материала.
Фантомы молочной железы с интегрированными элементами сферической формы
Это частный случай подхода, рассматриваемого в следующем подразделе. В гомогенную подлежащую ткань погружаются сферические элементы различной величины, изготовленные из тканеэквивалентного материала [46–48]. Данные фантомы широко используются для оценки качества изображений и измерения дозы облучения при ММГ, а также для оптимизации процедур сканирования в новых ММГ методиках, таких как двухэнергетическая ММГ, двухэнергетическая ММГ с контрастным усилением, томосинтез и КТ. Группа специалистов из отделения радиологии Лёвенского католического университета разработала фантом МЖ, состоящий из двух основных частей: акрилового полуцилиндрического контейнера, имитирующего МЖ сжатой формы толщиной 58 мм, и равных объёмов акриловых сфер шести различных диаметров [49]. В качестве альтернативы пространство между сферами может быть заполнено водой — материалом, рентгеновские свойства которого близки к свойствам реальной ткани МЖ. Оценка была проведена на ММГ-системе с возможностью томосинтеза. Хотя картина изображения, полученная с помощью этих фантомов, не похожа на реальное изображение МЖ, эти фантомы очень просты в изготовлении и позволяют легко получать различные варианты подлежащей ткани. Этот физический фантом имеет воздушные пузырьки в верхней части, поскольку полное заполнение фантома водой вызывает некоторые трудности. Усовершенствование данного фантома возможно с использованием вычислительной модели для оптимизации вариантов наполнения фантома [50–52].
Аналогичные подходы были описаны K. Bliznakova и соавт. [53–55], согласно которым полуцилиндрические контейнеры, напечатанные из фотополимерной смолы или нейлона, заполняются сферами из фотополимерной смолы, а в качестве наполнителя используется парафин или животное сало. Одной из реализаций такого подхода является фантом МЖ небольшого размера, форма которого представляет собой полуцилиндр и изготовлена из фотополимера Formlabs White resin. В этот физический фантом помещаются 27 сфер из фотополимера Formlabs Grey resin радиусом от 6 до 13 мм с последующим добавлением животного жира. Фантом был использован для изучения фазово-контрастной визуализации МЖ [54] и для разработки модели синтетического наблюдателя для использования в контроле качества систем томосинтеза [56].
Сборка фантомов молочной железы из отдельно напечатанных элементов
Такой подход требует наличия цифровых моделей основных тканей МЖ: кожи, железистой, жировой тканей и различных новообразований и микрокальцинатов. Эти отдельные цифровые модели создаются либо на основе математических расчётов, реализованных в специальных компьютерных программах [15, 57], либо на основе сегментации медицинских изображений пациентов [24]. Каждая смоделированная ткань МЖ сохраняется в отдельном файле и далее подготавливается для 3D-печати. Примером реализации такого подхода является фантом, разработанный N.T. Dukov и соавт., в котором стереолитография используется для печати внешней формы, сети молочных протоков и новообразований, а печать методом послойного наплавления (FDM) с использованием нитей из ABS-пластика — для жировых отсеков [37, 53]. Решение об использовании этих печатных материалов основано на обширном экспериментальном исследовании [32, 34]. Фантом заполняется водой, для имитации микрокальцинатов авторы используют измельчённую в мелкий порошок яичную скорлупу. Отметим, что при этом сама МЖ не визуализируется. Необходимы дальнейшие исследования новых материалов для 3D-печати, которые смогут имитировать рентгеновские свойства всех типов тканей МЖ.
В 2016 г. была создана новая версия фантома со вставными элементами сферической формы [6]. Модели сегментированных вручную жировых отделов МЖ, полученные на основе КТ-изображений образца мастэктомии [22], были напечатаны на стереолитографическом 3D-принтере с использованием материала Formlabs Clear Resin. Далее они помещались в полуцилиндрический контейнер толщиной 48 мм, заполненный водой, с последующим получением ММГ-изображения на аппарате Siemens Mammomat (Siemens Healthineers, Германия). В результате экспериментальные изображения были лучше, чем исходная модель, однако слишком отличались от ММГ-изображений пациенток.
Дальнейшие усовершенствования фантома с отсеками направлены на повышение сходства с данными пациентов. Это может быть достигнуто использованием менее плотного печатного материала для повышения контрастности отсеков, использованием отсеков меньшего размера путём уменьшения их масштаба, а также доработкой метода сегментации отсеков. Недавно командой из Венского университета был разработан ещё один вариант сферического фантома, в котором модель включает сферы, изготовленные из материала VeroClear, а в качестве наполнителя используется парафиновое масло [58].
Печать цельного фантома молочной железы
Печать с использованием одного материала
Физический фантом UPenn [59] основан на математической модели МЖ Пенсильванского университета, описанной в предыдущем разделе [16–18]. Данная технология производства предполагает первоначальное сегментирование вокселей цифрового фантома на две составляющие: фиброгландулярную и жировую ткани. Первая изготавливается с помощью принтера PolyJet Eden500V (Stratasys, США) с использованием тканеэквивалентного материала с 50% содержанием железистой ткани (фотополимер FC-720). Печать производится послойно, чтобы сохранить доступ к пустым пространствам, которые затем заполняются эквивалентом жировой ткани. Срезы печатаются с воксельным разрешением 60 мкм. Затем пластины соединяются вместе для создания окончательного антропоморфного фантома. Первоначальная экспериментальная оценка ММГ и томосинтезных изображений этого физического фантома показывает его потенциал для использования как для качественной, так и для количественной оценки характеристик систем двух- и трёхмерной рентгеновской визуализации МЖ. ММГ-изображения, полученные с помощью этого фантома, визуально схожи с клиническими. Некоторые ограничения данной технологии связаны с нечёткостью границ структур, наличием остаточных воздушных пузырьков, которые видны на рентгенограммах, длительным временем изготовления и высокой стоимостью производства.
Похожий подход использовали J.G. Mainprize и соавт. [40, 60] при создании двухкомпонентного полноразмерного физического фантома МЖ. Цифровой фантом на основе вокселей [61] разделяется на четыре пластины для размещения различных вкладышей на разной высоте. В каждой пластине вырезается фиброгландулярный компонент, остаётся только жировой. Полученный жировой слой печатается с помощью принтера селективного лазерного спекания с разрешением 100 мкм из полиамида-12. Исследования на фантоме показали высокое сходство с данными по параметру избыточной энтропии, полученными на клинических полноценных цифровых ММГ.
Изображения КТ МЖ конкретной пациентки являются основой для создания двухкамерного антропоморфного фантома МЖ, предложенного N.D. Prionas и соавт. [62]. Железистая часть состоит из воды, а жировая — из полиэтилена. 3D-изображения сначала получают на специализированном КТ МЖ, затем производят устранение шума и сегментацию тканей на жировую и железистую. Процесс изготовления отличается от описанных выше тем, что фантомы изготавливаются на водоструйном станке с числовым программным управлением. Стопка сегментов МЖ изготавливается из листового сверхвысокомолекулярного полиэтилена толщиной 1,59 мм с помощью водоструйного аппарата, при этом осевая линия траектории инструмента определяется по краям тканевой структуры. Вокруг фантома МЖ из термопластика формуется внешний контейнер, толщина которого приблизительно соответствует толщине кожи. Контейнер со стопкой сегментов МЖ заполняется водой таким образом, чтобы воздушные пространства в фантоме, представляющие собой отсек железистой ткани, были заполнены водой.
Крупные участки железистой ткани этого фантома точно соответствуют исходным изображениям пациента. Преимуществом модели является её модульная конструкция, позволяющая вводить дополнительные объекты в любой отсек железистой ткани. Основные ограничения связаны как с технологией, так и с используемым материалом: толщина полиэтиленовой массы, а также промежутки между изготовленными сегментами МЖ привели к тому, что фантом оказался на 2,6 см длиннее исходной МЖ пациентки. Характеристики материала ограничивают применение фантома рентгеновскими методиками, где компрессия на МЖ не проводится. Другие незначительные проблемы связаны с наличием воздушных пузырьков в железистой ткани, которые могут быть удалены с помощью более сложных методов дегазации при сборке фантома. Еще одна небольшая проблема связана с процессом гидроабразивной резки, который также может привести к уменьшению внешнего жирового контура и расширению контуров железистой ткани.
Двухмерные ММГ-изображения пациента — ключевой источник информации в методике печати физических антропоморфных фантомов МЖ, предложенной и реализованной двумя исследовательскими группами [63, 64] в 2018 и 2019 годах. A. Badal и соавт. [63] разработали легко воспроизводимую методику изготовления антропоморфных моделей МЖ на основе двухмерных ММГ-изображений. Для изготовления объектов МЖ использовался струйный принтер Objet260 Connex3 (Stratasys, США) с печатными материалами VeroMagenta и VeroCyan. Выбор данной технологии печати хорошо обоснован обширным экспериментальным исследованием, в котором применялись три принтера на различных технологиях: стереолитографии, моделирования методом FDM и струйной печати [65].
Ключевой элемент этого подхода — программный скрипт mammoreplicator на языке программирования Python, который рассчитывает толщину напечатанного материала в зависимости от уровня серого цвета каждого пикселя изображения и различий в поглощении рентгеновского излучения тканями МЖ и материалами для 3D-печати. Каждый пиксель изображения преобразуется скриптом в столбец соответствующей высоты.
Модель была напечатана за 10 ч, её масса составляет 750 г. В настоящее время она валидирована для корректного воспроизведения рентгенологических свойств МЖ при ММГ. Исследование сходства исходной и фантомной ММГ показало, что анатомические особенности воспроизводятся с хорошей точностью. Основное ограничение связано с разрешением полученного фантома. Хотя исходное разрешение ММГ пациента составляет 100 мкм, 3D-принтер не смог точно воспроизвести детали на клинических ММГ размером менее 300 мкм. Авторы предлагают использовать эту технологию для создания коллекций репрезентативных моделей пациентов, которые могут быть использованы для оценки влияния анатомической изменчивости МЖ на надёжность диагностической системы.
В работе S. Schopphoven и соавт. [64] использовалось ММГ-изображение МЖ, сжатой до 32 мм. Ключевая задача была связана с определением интенсивностей пикселей необработанных изображений для различной толщины печатного материала (полипропилена) для разнообразия диапазона клинических изображений. Это реализуется путём сканирования печатных пластин различной толщины (до 40 мм) на ММГ-установке. Соответствующее ослабление в позиции пикселя клинического изображения моделируется разностью высот материала для печати, в результате чего на фантоме образуется рельефоподобная структура.
Время печати фантома составило около 11 ч при расходе 791 г материала RGD450 и 31 г вспомогательного материала. Описанный подход позволяет создавать антропоморфные фантомы для ММГ, реалистично имитирующие анатомию и характеристики плотности МЖ, и может быть использован для решения различных задач контроля качества и оптимизации систем, а также в образовательных и научных целях. Основные ограничения, существующие в настоящее время, связаны с несколько сниженным разрешением при изображении мелких деталей по сравнению с исходным клиническим изображением. Это связано с выбранной комбинацией принтера и материала (принтер PolyJet в сочетании с полипропиленовым материалом для печати), которая ограничивает размер выводимых структур величиной примерно 200 мкм. Структуры располагаются на верхней части фантомов и, таким образом, находятся выше над детектором, чем структуры в МЖ пациента. Это может привести к усилению резкости на ММГ изображениях более плотных МЖ за счёт геометрического увеличения.
Моделирование скоплений микрокальцинатов в этом фантоме осуществляется с помощью 3D-печатной пластины-основания диаметром 4 мм с подвижными вставками и измельчённой яичной скорлупы [38]. Основание и вставки были напечатаны с помощью 3D-принтера Objet30 Pro PolyJet и материала VeroClear RGD810 (Stratasys, США). Пластина-основа содержала три круглые полости диаметром 60 мм, в которые отдельно помещались кольца, пронумерованные от «1» до «3». Первое кольцо — «кольцо поражения» — содержит три круглые полости диаметром 10 мм с соответствующими крышками, в которые помещены моделируемые микрокальцинаты. Два других кольца смоделированы сплошными и не содержат никаких структур. Все три кольца имеют одинаковые размеры, что обеспечивает их взаимозаменяемость и возможность изменять позицию очагов поражения относительно антропоморфного фантома. Различные скопления микрокальцинатов моделируются с помощью разного количества и размера яичной скорлупы.
Преимуществом данного подхода является модульная конструкция, которая позволяет просто и с минимальными затратами интегрировать дополнительные пластины и кольца с различными повреждениями в зависимости от целей использования. Фантомы, изготовленные по данной технологии, предназначены для использования в 2D-ММГ. Для трёхмерной визуализации МЖ очень важно, чтобы физические фантомы имитировали пространственное распределение различных тканей МЖ в трёх измерениях. Это может быть выполнено с помощью одного из подходов, рассмотренных ниже.
В 2018 году N. Okkalidis и соавт. [66] предложили новую методику одновременной печати фантомов МЖ непосредственно с данных КТ пациента по технологии моделирования методом FDM — с помощью изменения плотности заполнения однородного шаблона. Этот шаблон используется для точной имитации внутренней структуры моделируемого 3D-объекта. Наиболее толстые части объекта изготавливаются с максимальной плотностью заполнения 100%, а более мягкие и лёгкие ткани печатаются с меньшей плотностью. Это позволяет реалистично и с учётом рентгенологических особенностей создавать различные типы тканей. Метод основан на считывании показателей единиц Хаунсфилда с каждого вокселя, и на основании этого позволяет регулировать скорость экструзии нити полимолочной кислоты для получения необходимого количества экструдированной нити. Метод был применён при печати антропоморфного фантома МЖ непосредственно по данным КТ пациента [67]. Первая оценка фантома на обычном компьютерном томографе показала визуальное сходство исходных срезов КТ пациента и фантома, что мотивировало команду на разработку специализированного фантома для КТ-исследований и, в частности, на создание будущей экспериментальной установки для точной дозиметрии КТ. Основные ограничения — длительное время печати (несколько дней) и видимость напечатанного рисунка, обусловленная использованной в исследовании технологии моделирования FDM.
Печать с использованием двух и более материалов
Для создания идеальных антропоморфных фантомов МЖ оптимальным является использование различных печатных материалов. Большой шаг к реализации этой цели был сделан в 2015 году N. Kiarashi и соавт. [68], которым удалось изготовить антропоморфный фантом сжатой МЖ методом 3D-печати с использованием двух материалов параллельно [26, 69]. На 3D-принтере Objet500 Connex были напечатаны две антропоморфные модели МЖ. Первый фантом, получивший название Doublet, был напечатан с использованием одновременно двух материалов, моделирующих железистую и жировую ткани. Для печати фиброгландулярной части использовались материалы TangoGray, VeroWhite. В качестве материалов-наполнителей, приближающихся по рентгеновской плотности к жировой ткани, исследовались смесь сливочного масла и сала в отношении 1:1, пчелиный воск, смола, оливковое масло. Метод со смолой повторялся несколько раз с использованием различных техник заполнения, но всегда приводил к появлению некоторого количества нежелательных воздушных пузырьков.
Физические фантомы имеют реалистичный рентгенографический вид анатомии МЖ как на 2D, так и на 3D-изображениях. Описание ММГ физических фантомов соответствует реальным ММГ человека [70, 71]. Недостатки фантомов связаны с ограниченным динамическим диапазоном и контрастностью, обусловленными отсутствием подходящих материалов для печати. Эта проблема должна быть решена с появлением новых материалов. Несмотря на ограничения в контрастности, фантом Doublet обладает ключевым преимуществом — это конструкция «всё в одном», когда фантом (целиком или по частям) может быть изготовлен за один подход.
Дальнейшее развитие описанного выше подхода к печати антропоморфных моделей МЖ для имитации анатомии пациента с целью оценки эффективности клинической ММГ и цифрового томосинтеза МЖ описано в работе A.H. Rossman и соавт. [72]. Они создали модульный фантом с антропоморфной областью, позволяющей улучшить выявление очагов поражения и кальцинатов, а также однородной областью для оценки стандартных показателей контроля качества. Для печати фиброгландулярной ткани использовался материал VeroPureWhite и специальная смола Jf Flexible, легированная вольфрамом, а для изображения жировой ткани низкой плотности — материал TangoPlus и смола Jf Flexible стороннего производителя, без легирующего компонента. В конструкции предусмотрена возможность добавления образований, йодированных включений и кальцинатов. Одним из текущих ограничений данного фантома является невозможность достижения рентгенологической плотности МЖ по BI-RADS ниже 36%. От рентгенологической плотности зависит чувствительность изображения: чем выше плотность, тем ниже чувствительность ММГ. В настоящее время усилия направлены на достижение более низкой плотности.
В 2019 г. группа из Неаполя [73] впервые использовала 3D-принтер FDM для изготовления физических фантомов как несжатой, так и сжатой МЖ, имитирующих форму, а также анатомические и рентгенологические свойства реальных тканей МЖ. Для изготовления физического антропоморфного фантома МЖ использовались цифровые фантомы на основе данных КТ МЖ и три различных материала для печати. Поливинилацетат использовался для печати кожи, ABS-пластик и нейлон — для печати жировой и железистой тканей соответственно. Кожные покровы печаталась отдельно, поскольку вычислительные фантомы МЖ изготавливаются из трёх материалов, а имеющийся FDM-принтер Ultimaker 3 (Ultimaker, Нидерланды) печатает объекты максимум из двух материалов. Модели имеют 100% плотность заполнения для максимального уменьшения воздушных зазоров. Внутренняя область МЖ может быть представлена как в виде печатных срезов толщиной 10 мм, так и в виде единого блока из двухкомпонентного материала. Кроме того, авторы разработали область в печатных срезах для размещения TLD-чипов в дозиметрических целях, а также специальные отверстия для размещения печатных патологических очагов [73, 74].
Бумажные антропоморфные модели молочной железы
Бюджетный способ изготовления фантомов МЖ — использование офисной бумаги для печати и обычного струйного принтера [75]. В качестве добавок к краске могут быть использованы некоторые рентгеноконтрастные вещества, такие как йодид калия, оксид титана, рентгеноконтраст. Пергаментная бумага также была исследована, и оказалось, что она обладает сходной с жировой тканью рентгеновской плотностью. Физический фантом изготавливается на основе цифровой модели, представленной C.G. Graff и соавт. [21], в виде срезов методом струйной печати с использованием пергаментной бумаги и рентгеноконтрастных чернил, содержащих 33% или 25% йогексола по объёму. Преимуществом такого подхода к изготовлению антропоморфных фантомов является их низкая цена, поскольку для их производства используются имеющиеся в продаже струйные принтеры, недорогие улучшающие компоненты для чернил и некоторые дешёвые дополнительные материалы. В качестве струйного принтера в данном исследовании использовался серийно выпускаемый настольный струйный принтер Epson Workforce 630 (Epson, Япония). Вместо оригинальных картриджей производителя использовались многоразовые картриджи. Поскольку принтер имеет несколько цветных картриджей, можно печатать дополнительные «ткани» разными цветами. Например, для печати кожи можно определить соответствующую концентрацию йодных чернил и поместить их в отдельный картридж. Затем на кожу и железистую ткань изображения можно наносить различные цвета и печатать соответствующим образом.
Подводя итог, технология 3D-печати — хорошо изученная методика изготовления антропоморфных фантомов МЖ для использования в рентгеновской визуализации [68, 72]. Основное преимущество 3D-печати заключается в том, что она позволяет разрабатывать и печатать сложные конструкции. Тем не менее, некоторые особенности процесса 3D-печати ограничивают его универсальность и целесообразность [41, 76, 77]. Хотя 3D-печать позволяет создавать изделия из различных пластмасс и силиконов, не все они поддаются температурному воздействию, необходимому для 3D-печати. Кроме того, 3D-принтеры имеют небольшие камеры для печати, что ограничивает размер получаемых элементов. С практической точки зрения 3D-печать — это дорогостоящий и медленный процесс, а значит, нецелесообразный для крупносерийного производства. Медленность обусловлена тем, что время, необходимое для создания антропоморфных фантомов с помощью 3D-печати, зависит от сложности конструкции (желаемое количество органических тканей и элементов, которые должны быть включены в структуру фантома, а также желаемый размер). Кроме того, часто возникает необходимость в постобработке 3D-отпечатанных фантомов для удаления вспомогательного материала из конструкции и сглаживания поверхности для достижения требуемой финишной обработки. Она может включать в себя гидроабразивную обработку, выдержку в химических составах и воздушно-тепловую сушку. Каждый из этих вариантов связан с риском повреждения фантомов или появления в них нежелательных частиц, которые могут повлиять на результаты рентгеновской визуализации.
Альтернативным подходом к созданию физических фантомов является послойное создание модели. Каждый слой представляет собой планарное сечение. При таком подходе сложность создания фантома сводится к обработке двухмерных слоёв. Впервые этот подход был представлен C. Theodorakou и соавт. [78]. В упомянутой работе каждый слой фантома состоял из подложки и контрастного материала (красителя), наносимого на подложку с помощью стандартного струйного принтера. С тех пор несколько исследовательских групп развили эту концепцию и сообщили об успешных реализациях [79, 80]. Процесс послойного создания фантома, хотя и позволяет детально формировать свойства рентгеновской плотности путём добавления красителей на двухмерный слой, имеет ряд присущих ему ограничений. В основном эти ограничения связаны с зависимостью от использования контрастных материалов (красителей). Помимо сложностей, связанных с оптимизацией химического состава красителей, контрастный материал часто находится в жидком состоянии, а подложка — в твёрдом. Нанесение жидкого контрастного материала на твёрдую подложку часто приводит к просачиванию красителя в подложку или его растеканию по подложке, что влечёт за собой трудности в управлении позиционированием контрастного материала. Кроме того, при таком подходе обычно требуется многослойное нанесение красителей на одну подложку, что приводит к многоступенчатой сушке, негативно влияющей на время изготовления. Наконец, компоненты струйных принтеров часто не выдерживают многократной подачи красителей. В струйных принтерах используются небольшие сопла для подачи чернил. Некоторые контрастные материалы, например, соединения на основе йода, широко используемые в рентгеновской визуализации, часто приводят к засорению сопел, что требует больших затрат времени и труда на техническое обслуживание для устранения сбоев в производственном процессе.
Краткий обзор коммерческих фантомов
Фантом молочной железы для маммографии и томосинтеза, разработанный Erler Zimmer и реализуемый компанией GTSimulators
Процесс создания этого коммерческого фантома состоит из двух этапов. В начале происходит создание расчётной модели МЖ по методике C.G. Graff [21]. Поверхность МЖ формируют путём создания оболочки в виде квадратичной полусферы, на которую накладывают слой кожи и сосок. Форма оболочки определяется с помощью двух параметров: определяющих общий объём МЖ и корректирующих кривизну поверхности. С помощью сегментации Вороного внутренняя часть оболочки случайным образом делится на жировой и железистый компоненты, причём каждый железистый компонент содержит сеть протоков с терминальными дольковыми единицами. Затем объём заполняется дополнительными элементами МЖ, такими как связки Купера, грудные мышцы и кровеносные сосуды. Кроме того, смоделировано злокачественное новообразование [81], которое впоследствии введено в модель.
Для создания компрессии МЖ её объём был преобразован в четырёхмерную матрицу, и каждому элементу этой матрицы были приданы упругие свойства, определяемые вокселями железистой или жировой ткани, расположенными в центре элемента. Затем с помощью линейно-упругого конечно-элементного моделирования была сформирована матрица, и МЖ была сжата в кранио-каудальном направлении до толщины 30 мм. Однако нет никаких ограничений для создания фантома с любой толщиной МЖ. Поскольку модель фантома основана на аналитических выражениях, толщина сжатия и размер вокселя произвольны; в данном исследовании модель МЖ была дискретизирована с размером изотропного вокселя 70 мкм, что соответствует толщине пергаментной бумаги. В зависимости от размера элементов детектора при заданном размере вокселя могут возникать нежелательные эффекты, связанные с дискретизацией изображения.
Второй этап – создание физического фантома методом струйной печати. В качестве принтера использовался серийно выпускаемый настольный струйный принтер (Epson Workforce 630). Разрешение печати было установлено на 363 точки на дюйм, чтобы обеспечить размер точек 70 мкм и соответствовать размеру вокселей цифрового фантома (70 мкм). В этой процедуре на бумагу, служащую в качестве фоновой жировой ткани, наносились специализированные чернила, создающие сходные с железистой тканью характеристики плотности. Чернила синтезировались путём смешивания обычных пигментных чернил (InkThrift, Vermont PhotoInkjet, East Topsham Village, VT) с 350 мг/мл йогексола (Omnipaque, GE Healthcare, США). В одном растворе — 67% чернил и 33% йогексола, в другом — 75% чернил и 25% йогексола. Для выравнивания листов был создан специализированный дырокол для проделывания отверстий над фидуциальными маркёрами, листы затем скользили по стойкам, прикреплённым к специализированной опорной пластине для обеспечения неподвижности листов после размещения. После того как отпечатанные листы были сложены на опорной плите, сверху на стопку помещалась большая специализированная компрессионная лопатка с отверстиями для стоек, чтобы зафиксировать листы и обеспечить равномерное сжатие.
Для демонстрации возможностей фантома после изготовления всей стопки листов в него были включены трёхмерные очаги поражения размером около 5 мм и кластеры, содержащие микрокальцинаты. Трёхмерный очаг поражения сначала вводился виртуально, а затем помещался в физический фантом путём перепечатки выбранных срезов, на которые он распространялся, и подстановки их на место листов без очага поражения в соответствующих местах. Микрокальцинаты моделировались путём дробления яичной скорлупы и размещения её в местах различной плотности желёз на физическом фантоме МЖ. Количество элементов в одном кластере варьировало от 11 до >30. Диаметр кластеров находился в диапазоне от 3,5 мм до 8 мм.
Использованные материалы схожи с жировой и железистой тканями по показателям рентгеновской плотности, а процесс изготовления является точным, воспроизводимым и может быть использован как для 2D, так и для 3D-визуализации МЖ. Кроме того, данный фантом не ограничивается одной моделью МЖ, и любая виртуальная модель, будучи вокселизированной, может быть напечатана с использованием этого уникального подхода.
Данный фантом прошёл процесс валидации [75] и использовался в исследовании возможностей свёрточных нейронных сетей в дифференциальной диагностике доброкачественных и злокачественных кальцинатов при обычной и двухэнергетической полноформатной цифровой ММГ [84].
BR3D Breast Imaging Phantom
Данный фантом описан выше в разделе «Фантомы молочной железы, изготовленные с использованием технологии литья» и представлен на рис. 3.
Рис. 3. BR3D Breast Imaging Phantom [82].
Модель валидирована и использована в многочисленных исследованиях:
- Исследование, посвящённое оптимизации изображений цифрового томосинтеза МЖ на основе моделей для итеративной реконструкции [83]. Новый алгоритм реконструкции изображений для цифрового томосинтеза МЖ, реализованный с использованием регуляризатора Total Variation, был проверен на фантоме Model 20 BR3D. Полученные результаты подтверждают способность данного алгоритма к точному изображению микрокальцинатов и образований МЖ.
- Оценка ускорения графического процессора итерационного метода на основе моделей для цифрового томосинтеза МЖ [84]. В исследовании показано, что параллельная обработка, реализованная на трёх различных платах графического процессора, позволяет быстро реализовать итеративную реконструкцию изображений, полученных с помощью цифрового томосинтеза МЖ.
- Оценка генеративно-состязательной сети для улучшения качества изображения и снижения дозы облучения при цифровом томосинтезе МЖ [85]. Продемонстрировано улучшение качества изображения при цифровом томосинтезе МЖ в условиях низких доз облучения при предварительной обработке реконструкции с использованием условных генеративно-состязательных сетей [cGAN (pix2pix)].
Другие фантомы молочной железы
На рынке существуют коммерческие фантомы МЖ, данные об использовании которых в научных исследованиях недоступны. Среди них:
- Модель 011A производителя CIRS (США) представляет собой тканеэквивалентный антропоморфный фантом, предназначенный для проверки работоспособности всех видов ММГ-систем. В качестве тестовых объектов в фантом встраиваются модели кальцификатов, протоков и новообразований. Размеры тест-объектов варьируют, что позволяет проверять систему на различном уровне сложности. Материал смолы имитирует коэффициенты ослабления фотонов различных тканей МЖ. Средний элементный состав имитируемой ткани основан на индивидуальном элементном составе жировой и железистой тканей. Данный фантом применялся в ряде исследований, направленных на повышение точности диагностических методов [86–88]. Фантом представлен на рис. 4.
- BT-A01 производства True Phantom Solutions (Канада) — изображение женского полуторса в натуральную величину, тщательно проработанное с учётом типичных анатомических особенностей. Используется для ММГ, КТ, МРТ. Целевая аудитория — медицинские работники и студенты.
- Complex Breast Phantom SynAtomy 160650 производства SynDaver (США) — антропоморфный фантом МЖ, предназначен для обучения студентов и медицинских сотрудников. Модальности: ультразвуковое исследование, эластография, ММГ.
Рис. 4. Модель 011A производителя CIRS.
ЗАКЛЮЧЕНИЕ
В данной статье представлен краткий исторический экскурс в разработку и применение антропоморфных моделей МЖ, предназначенных для рентгеновской визуализации. Различные подходы к созданию таких фантомов имеют как преимущества, так и ограничения. В зависимости от конкретных задач медицинской визуализации предпочтение может быть отдано тому или иному подходу. В то время как вычислительные антропоморфные фантомы обладают анатомической достоверностью и относительной простотой моделирования, изготовление физических антропоморфных моделей сопряжено с трудностями. Имеющиеся технологии затратны и трудоёмки, а диапазон используемых материалов ограничен. Несмотря на то, что в этой области уже проделана большая работа, необходим поиск новых материалов, обладающих коэффициентами поглощения рентгеновского излучения, соответствующими различным типам тканей МЖ. Кроме того, необходимо разрабатывать и внедрять недорогие технологии производства. Это позволит улучшить и ускорить изготовление антропоморфных фантомов МЖ для рентгеновской визуализации.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Данная статья подготовлена авторским коллективом в рамках научно-исследовательской работы «Научное обоснование разработки и применения тканеэквивалентных материалов для создания тест-объектов в области лучевой диагностики» (№ в Единой государственной информационной системе учёта: 123092000013-3 ) в соответствии с Приказом от 21.12.2022 г. № 1196 «Об утверждении государственных заданий, финансовое обеспечение которых осуществляется за счёт средств бюджета города Москвы государственным бюджетным (автономным) учреждениям подведомственным Департаменту здравоохранения города Москвы, на 2023 год и плановый период 2024 и 2025 годов» Департамента здравоохранения города Москвы.
Конфликт интересов. Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов: Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение поисково-аналитической работы и подготовку статьи, прочли и одобрили финальную версию перед публикацией).
Наибольший вклад распределён следующим образом: Ю.А. Васильев — концепция и дизайн исследования; анализ материала; утверждение окончательного варианта статьи; О.В. Омелянская — концепция и дизайн исследования; редактирование; утверждение окончательного варианта статьи; А.А. Насибуллина — написание текста; сбор и обработка материала; анализ материала; Д.В. Леонов — сбор и обработка материала; анализ материала; редактирование; Ю.В. Булгакова — написание текста; анализ материала; Д.А. Ахмедзянова, Ю.Ф. Шумская — написание текста; сбор и обработка материала; обзор литературы; Р.В. Решетников — концепция и дизайн исследования; обзор литературы; редактирование; утверждение окончательного варианта статьи.
Об авторах
Юрий Александрович Васильев
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: VasilevYA1@zdrav.mos.ru
ORCID iD: 0000-0002-5283-5961
SPIN-код: 4458-5608
канд. мед. наук
Россия, МоскваОльга Васильевна Омелянская
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: OmelyanskayaOV@zdrav.mos.ru
ORCID iD: 0000-0002-0245-4431
SPIN-код: 8948-6152
Россия, Москва
Анастасия Александровна Насибуллина
Научно-практический клинический центр диагностики и телемедицинских технологий
Автор, ответственный за переписку.
Email: NasibullinaAA@zdrav.mos.ru
ORCID iD: 0000-0003-1695-7731
SPIN-код: 2482-3372
Россия, Москва
Денис Владимирович Леонов
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: LeonovDV2@zdrav.mos.ru
ORCID iD: 0000-0003-0916-6552
SPIN-код: 5510-4075
канд. техн. наук
Россия, МоскваЮлия Владиславовна Булгакова
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: BulgakovaYV@zdrav.mos.ru
ORCID iD: 0000-0002-1627-6568
SPIN-код: 8945-6205
Россия, Москва
Дина Альфредовна Ахмедзянова
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: AkhmedzyanovaDA@zdrav.mos.ru
ORCID iD: 0000-0001-7705-9754
SPIN-код: 6983-5991
Россия, Москва
Юлия Федоровна Шумская
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: shumskayayf@zdrav.mos.ru
ORCID iD: 0000-0002-8521-4045
SPIN-код: 3164-5518
Россия, Москва
Роман Владимирович Решетников
Научно-практический клинический центр диагностики и телемедицинских технологий
Email: r.reshetnikov@npcmr.ru
ORCID iD: 0000-0002-9661-0254
SPIN-код: 8592-0558
канд. ф.-м. наук
Россия, МоскваСписок литературы
- Leonov D., Venidiktova D., Costa-Júnior J.F.S., et al. Development of an anatomical breast phantom from polyvinyl chloride plastisol with lesions of various shape, elasticity and echogenicity for teaching ultrasound examination // International Journal of Computer Assisted Radiology and Surgery. 2023. doi: 10.1007/s11548-023-02911-4
- Nuzov N.B., Bhusal B., Henry K.R., et al. True location of deep brain stimulation electrodes differs from what is seen on postoperative magnetic resonance images: An anthropomorphic phantom study // Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. 2022. P. 1863–1866. doi: 10.1109/EMBC48229.2022.9871619
- Cannella R., Shahait M., Furlan A.A., et al. Efficacy of single-source rapid kV-switching dual-energy CT for characterization of non-uric acid renal stones: a prospective ex vivo study using anthropomorphic phantom // Abdominal Radiology. 2020. Vol. 45, N 4. P. 1092–1099. doi: 10.1007/s00261-019-02164-3
- Kramer R., Zankl M., Williams G., Drexler G., et al. The calculation of dose from external photon exposures using reference human phantoms and Monte Carlo methods. 1982.
- Васильев Ю.А., Тыров И.А., Владзимирский А.В., и др. Двойной просмотр результатов маммографии с применением технологий искусственного интеллекта: новая модель организации массовых профилактических исследований // Digital Diagnostics. 2023. Т. 4, № 2. C. 93–104. doi: 10.17816/DD3214236.
- Cockmartin L., Bosmans H., Marshall N.W. Comparative power law analysis of structured breast phantom and patient images in digital mammography and breast tomosynthesis // Med Phys. 2013. Vol. 40, № 8. P. 81920.
- Ma A.K.W., Gunn S., Darambara D.G. Introducing DeBRa: a detailed breast model for radiological studies // Physics in medicine and biology. 2009. Vol. 54, N 14. P. 4533–4545. doi: 10.1088/0031-9155/54/14/010
- Chen B., Shorey J., Saunders R.S., et al. An Anthropomorphic Breast Model for Breast Imaging Simulation and Optimization // Academic radiology. 2011. Vol. 18, N 5. P. 536–546. doi: 10.1016/j.acra.2010.11.009
- Elangovan P., Mackenzie A., Dance D.R., et al. Design and validation of realistic breast models for use in multiple alternative forced choice virtual clinical trials // Physics in medicine and biology. 2017. Vol. 62, N 7. P. 2778–2794. doi: 10.1088/1361-6560/aa622c
- Bliznakova K., Suryanarayanan S., Karellas A., Pallikarakis N. Evaluation of an improved algorithm for producing realistic 3D breast software phantoms: Application for mammography // Medical Physics. 2010. Vol. 37, N 11. P. 5604–5617. doi: 10.1118/1.3491812
- O’Connor J.M., Das M., Dider C., Mahd M., Glick S.J. Generation of voxelized breast phantoms from surgical mastectomy specimens // Medical Physics. 2013. Vol. 40, N 4. doi: 10.1118/1.4795758
- Lau B.A., Reiser I., Nishikawa R.M. A statistically defined anthropomorphic software breast phantom // Medical Physics. 2012. Vol. 39, N 6. P. 3375–3385. doi: 10.1118/1.4718576
- Sarno A., Mettivier G., di Franco F., et al. Dataset of patient-derived digital breast phantoms for in silico studies in breast computed tomography, digital breast tomosynthesis, and digital mammography // Medical Physics. 2021. Vol. 48, N 5. P. 2682–2693. doi: 10.1002/mp.14826
- Li C.M., Segars W.P., Tourassi G.D., Boone J.M., Dobbins J.T. Methodology for generating a 3D computerized breast phantom from empirical data // Medical Physics. 2009. Vol. 36, N 7. P. 3122–3131. doi: 10.1118/1.3140588
- Bliznakova K., Bliznakov Z., Bravou V., Kolitsi Z., Pallikarakis N. A three-dimensional breast software phantom for mammography simulation // Physics in medicine and biology. 2003. Vol. 48, N 22. P. 3699–3719. doi: 10.1088/0031-9155/48/22/006
- Bakic P.R., Albert M., Brzakovic D., Maidment A.D. Mammogram synthesis using a 3D simulation. I. Breast tissue model and image acquisition simulation // Medical Physics. 2002. Vol. 29, N 9. P. 2131–2139. doi: 10.1118/1.1501143
- Bakic P.R., Albert M., Brzakovic D., Maidment A.D. Mammogram synthesis using a 3D simulation. II. Evaluation of synthetic mammogram texture // Medical Physics. 2002. Vol. 29, N 9. P. 2140–2151. doi: 10.1118/1.1501144
- Bakic P.R., Albert M., Brzakovic D., Maidment A.D. Mammogram synthesis using a three-dimensional simulation. III. Modeling and evaluation of the breast ductal network // Medical Physics. 2003. Vol. 30, N 7. P. 1914–1925. doi: 10.1118/1.1586453
- Pokrajac D.D., Maidment A.D.A., Bakic P.R. Optimized generation of high resolution breast anthropomorphic software phantoms // Medical Physics. 2012. Vol. 39, N 4. P. 2290–2302. doi: 10.1118/1.3697523
- Chen F., Pokrajac D., Shi X., et al. Partial volume simulation in software breast phantoms // Medical Imaging 2012: Physics of Medical Imaging. 2012. doi: 10.1117/12.912242
- Graff C.G. A new, open-source, multi-modality digital breast phantom // Proceedings of the SPIE. 2016. Vol. 9783. doi: 10.1117/12.2216312
- Ikejimba L.C., Salad J., Graff C.G., et al. A four-alternative forced choice (4AFC) methodology for evaluating microcalcification detection in clinical full-field digital mammography (FFDM) and digital breast tomosynthesis (DBT) systems using an inkjet-printed anthropomorphic phantom // Medical Physics. 2019. Vol. 46, N 9. P. 3883–3892. doi: 10.1002/mp.13629
- Imran A.-A.-Z., Bakic P.R., Pokrajac D.D. Spatial distribution of adipose compartments size, shape and orientation in a CT breast image of a mastectomy specimen // 2015 IEEE Signal Processing in Medicine and Biology Symposium (SPMB). 2015. P. 1–2. doi: 10.1109/SPMB.2015.7405460
- Imran A.-A.-Z., Pokrajac D.D., Maidment A.D.A., Bakic P.R. Estimation of adipose compartment volumes in CT images of a mastectomy specimen // Proceedings of the SPIE. 2016. Vol. 9783. doi: 10.1117/12.2217175
- Hoeschen C., Fill U., Zankl M., et al. A high-resolution voxel phantom of the breast for dose calculations in mammography // Radiation protection dosimetry. 2005. Vol. 114, N 1–3. P. 406–409. doi: 10.1093/rpd/nch558
- Hsu C.M., Palmeri M.L., Segars W.P., Veress A.I., Dobbins J.T. An analysis of the mechanical parameters used for finite element compression of a high-resolution 3D breast phantom // Medical Physics. 2011. Vol. 38, N 10. P. 5756–5770. doi: 10.1118/1.3637500
- Hsu C.M.L., Palmeri M.L., Segars W.P., Veress A.I., Dobbins J.T. Generation of a suite of 3D computer-generated breast phantoms from a limited set of human subject data // Medical Physics. 2013. Vol. 40, N 4. doi: 10.1118/1.4794924
- Huang S.Y., Boone J.M., Yang K., et al. The characterization of breast anatomical metrics using dedicated breast CT // Medical Physics. 2011. Vol. 38, N 4. P. 2180–2191. doi: 10.1118/1.3567147
- Segars W.P., Veress A.I., Wells J.R., et al. Population of 100 realistic, patient-based computerized breast phantoms for multi-modality imaging research // Proceedings of the SPIE. 2014. Vol. 9033. doi: 10.1117/12.2043868
- Erickson D.W., Wells J.R., Sturgeon G.M., et al. Population of 224 realistic human subject-based computational breast phantoms // Medical Physics. 2015. Vol. 43, N 1. P. 23–32. doi: 10.1118/1.4937597
- Sarno A., Mettivier G., Di Lillo F., et al. Homogeneous vs. patient specific breast models for Monte Carlo evaluation of mean glandular dose in mammography // Physica Medica. 2018. Vol. 51. P. 56–63. doi: 10.1016/j.ejmp.2018.04.392
- Ivanov D., Bliznakova K., Buliev I., et al. Suitability of low density materials for 3D printing of physical breast phantoms // Physics in medicine and biology. 2018. Vol. 63, N 17. doi: 10.1088/1361-6560/aad315
- Santos J.C., Almeida C.D., Iwahara A., Peixoto J.E. Characterization and applicability of low-density materials for making 3D physical anthropomorphic breast phantoms // Radiation Physics and Chemistry. 2019. Vol. 164. doi: 10.1016/j.radphyschem.2019.108361
- Esposito G., Mettivier G., Bliznakova K., et al. Investigation of the refractive index decrement of 3D printing materials for manufacturing breast phantoms for phase contrast imaging // Physics in medicine and biology. 2019. Vol. 64, N 7. doi: 10.1088/1361-6560/ab0670
- Bliznakova K., Buliev I., Bliznakov Z. Anthropomorphic Phantoms in Image Quality and Patient Dose Optimization. Philadelphia : IOP Publishing, 2018. doi: 10.1088/2053-2563/aae197
- Hernandez A.M., Seibert J.A., Nosratieh A., Boone J.M. Generation and analysis of clinically relevant breast imaging x-ray spectra // Medical Physics. 2017. Vol. 44, N 6. P. 2148–2160. doi: 10.1002/mp.12222
- Dukov N.T., Feradov F.N., Gospodinova G.D., Bliznakova K.S. An Approach for Printing Tissue-mimicking Abnormalities Dedicated to Applications in Breast Imaging // 2019 IEEE XXVIII International Scientific Conference Electronics (ET). 2019. P. 1–4. doi: 10.1109/ET.2019.8878587
- Mäder U., Martin F., Karin B., Stephan S. Concept to extend anthropomorphic breast phantoms for 2D digital mammography with movable lesions at variable reproducible positions // 15th International Workshop on Breast Imaging (IWBI2020). 2020. doi: 10.1117/12.2560619
- Okoh F.O., Kabir N.A., Mohd F.M.Y., Siti N.A.A. Measurement of mass attenuation coefficient of polyvinyl alcohol (PVAL) as breast tissue equivalent material in the photon energy range of 16.61–25.26 keV // Journal of Physics: Conference Series. 2020. Vol. 1535, N 1. doi: 10.1088/1742-6596/1535/1/012051
- Mainprize J.G., Mawdsley G.E., Carton A.-K., et al. Full-size anthropomorphic phantom for 2D and 3D breast x-ray imaging // Proceedings of the SPIE. 2020. Vol. 11513. P. 17. doi: 10.1117/12.2560358
- Filippou V., Tsoumpas C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound // Medical Physics. 2018. Vol. 45, N 9. P. e740–e760. doi: 10.1002/mp.13058
- di Franco F., Mettivier G., Sarno A., Varallo A., Russo P. Manufacturing of physical breast phantoms with 3D printing technology for X-ray breast imaging // 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). 2019. P. 1–5. doi: 10.1109/NSS/MIC42101.2019.9059986
- Sage J., Fezzani K.L., Fitton I., et al. Experimental evaluation of seven quality control phantoms for digital breast tomosynthesis // Physica Medica. 2019. Vol. 57. P. 137–144. doi: 10.1016/j.ejmp.2018.12.031
- Freed M., Badal A., Jennings R.J., et al. X-ray properties of an anthropomorphic breast phantom for MRI and x-ray imaging // Physics in medicine and biology. 2011. Vol. 56, N 12. P. 3513–3533. doi: 10.1088/0031-9155/56/12/005
- Ruvio G., Solimene R., Cuccaro A., et al. Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging // Sensors. 2020. Vol. 20, N 8. P. 2400. doi: 10.3390/s20082400
- Baldelli P., Phelan N., Egan G. Investigation of the effect of anode/filter materials on the dose and image quality of a digital mammography system based on an amorphous selenium flat panel detector // Br J Radiol. 2010. Vol. 83, N 988. P. 290–295. doi: 10.1259/bjr/60404532
- Park S., Jennings R., Liu H., Badano A., Myers K. A statistical, task-based evaluation method for three-dimensional x-ray breast imaging systems using variable-background phantoms // Medical Physics. 2010. Vol. 37, N 12. P. 6253–6270. doi: 10.1118/1.3488910
- Taibi A., Fabbri S., Baldelli P., et al. Dual-energy imaging in full-field digital mammography: a phantom study // Physics in medicine and biology. 2003. Vol. 48, N 13. P. 1945–1956. doi: 10.1088/0031-9155/48/13/307
- Cockmartin L., Marshall N., Bosmans H. Design and Evaluation of a Phantom with Structured Background for Digital Mammography and Breast Tomosynthesis. In: Maidment A.D.A., Bakic P.R., Gavenonis S., editors. Breast Imaging. IWDM 2012. Lecture Notes in Computer Science, vol 7361. Berlin : Springer, 2012. doi: 10.1007/978-3-642-31271-7_83
- Baneva Y., Bliznakova K., Cockmartin L., et al. Evaluation of a breast software model for 2D and 3D X-ray imaging studies of the breast // Physica Medica. 2017. Vol. 41. P. 78–86. doi: 10.1016/j.ejmp.2017.04.024
- Bliznakova K. Development of breast software phantom dedicated for research and educational purposes // RAD Association Journal. 2017. Vol. 2, N 1. P. 14–19. doi: 10.21175/RadJ.2017.01.004
- Marinov S., Carton A.-K., Cockmartin L., et al. Evaluation of the visual realism of breast texture phantoms in digital mammography // Proc. SPIE 11513, 15th International Workshop on Breast Imaging (IWBI2020). 2020. doi: 10.1117/12.2564124
- Feradov F., Marinov S., Bliznakova K. Physical Breast Phantom Dedicated for Mammography Studies. In: Henriques J., Neves N., de Carvalho P., editors. XV Mediterranean Conference on Medical and Biological Engineering and Computing – MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, 2020. P. 344–352. doi: 10.1007/978-3-030-31635-8_41
- Bliznakova K., Mettivier G., Russo P., Bliznakov Zh. Validation of a software platform for 2D and 3D phase contrast imaging: preliminary subjective evaluation // 15th International Workshop on Breast Imaging (IWBI2020). 2020. P. 97. doi: 10.1117/12.2564356
- Bliznakova K., Mettivier G., Russo P., et al. A software platform for phase contrast x-ray breast imaging research // Comput Biol Med. 2015. Vol. 61. P. 62–74. doi: 10.1016/j.compbiomed.2015.03.017
- Petrov D., Marshall N.W., Young K.C., Bosmans H. Systematic approach to a channelized Hotelling model observer implementation for a physical phantom containing mass-like lesions: Application to digital breast tomosynthesis // Physica Medica. 2019. Vol. 58. P. 8–20. doi: 10.1016/j.ejmp.2018.12.033
- Mettivier G., Bliznakova K., Sechopoulos I., et al. Evaluation of the BreastSimulator Software Platform for Breast Tomography: Preliminary Results // Physics in Medicine and Biology. 2016. Vol. 62, N 16. P. 145–151. doi: 10.1088/1361-6560/aa6ca3
- Salomon E., Semturs F., Unger E., et al. Equivalent breast thickness and dose sensitivity of a next iteration 3D structured breast phantom with lesion models // Medical Imaging 2020: Physics of Medical Imaging. 2020. doi: 10.1117/12.2548956
- Carton A.-K., Bakic P., Ullberg C., Derand H., Maidment A.D. Development of a physical 3D anthropomorphic breast phantom // Medical Physics. 2011. Vol. 38, N 2. P. 891–896. doi: 10.1118/1.3533896
- Mainprize J.G., Carton A.-K., Klausz R., et al. Development of a physical 3D anthropomorphic breast texture model using selective laser sintering rapid prototype printing // Medical Imaging 2018: Physics of Medical Imaging. 2018. P. 9. doi: 10.1117/12.2560358
- Li Z., Desolneux A., Muller S., Carton A.-K. A Novel 3D Stochastic Solid Breast Texture Model for X-Ray Breast Imaging. In: Tingberg A., Lång K., Timberg P., editors. Breast Imaging. IWDM 2016. Lecture Notes in Computer Science, vol 9699. Springer, 2016. P. 660–667. doi: 10.1007/978-3-319-41546-8_822016
- Prionas N.D., Burkett G.W., McKenney S.E., et al. Development of a patient-specific two-compartment anthropomorphic breast phantom // Physics in medicine and biology. 2012. Vol. 57, N 13. P. 4293–4307. doi: 10.1088/0031-9155/57/13/4293
- Badal A., Clark M., Ghammraoui B. Reproducing two-dimensional mammograms with three-dimensional printed phantoms // Journal of Medical Imaging. 2018. Vol. 5, N 3. doi: 10.1117/1.JMI.5.3.033501
- Schopphoven S., Cavael P., Bock K., Fiebich M., Mäder U. Breast phantoms for 2D digital mammography with realistic anatomical structures and attenuation characteristics based on clinical images using 3D printing // Physics in medicine and biology. 2019. Vol. 64, N 21. doi: 10.1088/1361-6560/ab3f6a
- Clark M., Ghammraoui B., Badal A. Reproducing 2D breast mammography images with 3D printed phantoms // Medical Imaging 2016: Physics of Medical Imaging. 2016. Vol. 9783. doi: 10.1117/12.2217215
- Okkalidis N. A novel 3D printing method for accurate anatomy replication in patient-specific phantoms // Medical Physics. 2018. Vol. 45, N 10. P. 4600–4606. doi: 10.1002/mp.13154
- Daskalov S., Okkalidis N., Boone J.M., et al. Anthropomorphic Physical Breast Phantom Based on Patient Breast CT Data: Preliminary Results. In: Henriques J., Neves N., de Carvalho P., editors. XV Mediterranean Conference on Medical and Biological Engineering and Computing – MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, 2020. P. 367–374. doi: 10.1007/978-3-030-31635-8_442020
- Kiarashi N., Nolte A.C., Sturgeon G.M., et al. Development of realistic physical breast phantoms matched to virtual breast phantoms based on human subject data // Medical Physics. 2015. Vol. 42, N 7. P. 4116–4126. doi: 10.1118/1.4919771
- Lindfors K.K., Boone J.M., Nelson T.R., et al. Dedicated Breast CT: Initial Clinical Experience // Radiology. 2008. Vol. 246, N 3. P. 725–733. doi: 10.1148/radiol.2463070410
- Burgess A.E., Judy P.F. Signal detection in power-law noise: effect of spectrum exponents // Journal of the Optical Society of America A. 2007. Vol. 24, N 12. P. B52–B60. doi: 10.1364/JOSAA.24.000B52
- Burgess A.E., Jacobson F.L., Judy P.F. Human observer detection experiments with mammograms and power-law noise // Medical Physics. 2001. Vol. 28, N 4. P. 419–437. doi: 10.1118/1.1355308
- Rossman A.H., Catenacci M., Zhao C., et al. Three-dimensionally-printed anthropomorphic physical phantom for mammography and digital breast tomosynthesis with custom materials, lesions, and uniform quality control region // Journal of Medical Imaging. 2019. Vol. 6, N 2. doi: 10.1117/1.JMI.6.2.021604
- Mettivier G., Sarno A., Boone J.M., et al. Virtual clinical trials in 3D and 2D breast imaging with digital phantoms derived from clinical breast CT scans // Medical Imaging 2020: Physics of Medical Imaging. 2020. doi: 10.1117/12.2548224
- Mettivier G., Sarno A, di Franco F., et al. The Napoli-Varna-Davis project for virtual clinical trials in X-ray breast imaging // 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). 2019. P. 1–5. doi: 10.1109/NSS/MIC42101.2019.9059828
- Ikejimba L.C., Graff C.G., Rosenthal S., et al. A novel physical anthropomorphic breast phantom for 2D and 3D x-ray imaging // Medical Physics. 2017. Vol. 44, N 2. P. 407–416. doi: 10.1002/mp.12062
- Mei K., Geagan M., Roshkovan L., et al. Three-dimensional printing of patient-specific lung phantoms for CT imaging: Emulating lung tissue with accurate attenuation profiles and textures // Medical Physics. 2022. Vol. 49, N 2. P. 825–835. doi: 10.1002/mp.15407
- Ionita C.N., Mokin M., Varble N., et al. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing // Proceedings of SPIE--the International Society for Optical Engineering. doi: 10.1117/12.2042266
- Theodorakou C., Horrocks J.A., Marshall N.W., Speller R.D. A novel method for producing x-ray test objects and phantoms // Physics in medicine and biology. 2004. Vol. 49, N 8. P. 1423–1438. doi: 10.1088/0031-9155/49/8/004
- Sikaria D., Musinsky S., Sturgeon G.M., et al. Second generation anthropomorphic physical phantom for mammography and DBT: Incorporating voxelized 3D printing and inkjet printing of iodinated lesion inserts. Proc. SPIE 9783, Medical Imaging 2016: Physics of Medical Imaging. 2016. doi: 10.1117/12.2217667
- Jahnke P., Limberg F.R., Gerbl A., et al. Radiopaque Three-dimensional Printing: A Method to Create Realistic CT Phantoms // Radiology. 2017. Vol. 282, N 2. P. 569–575. doi: 10.1148/radiol.2016152710
- de Sisternes L., Brankov J.G., Zysk A.M., et al. A computational model to generate simulated three-dimensional breast masses // Medical Physics. 2015. Vol. 42, N 2. P. 1098–1118. doi: 10.1118/1.4905232
- SUN NUCLEAR. BR3D BREAST IMAGING PHANTOM [Internet] [дата обращения 01.01.2023]. Доступ по ссылке: https://www.cirsinc.com/products/mammography/br3d-breast-imaging-phantom/
- Piccolomini E.L., Morotti E. A Model-Based Optimization Framework for Iterative Digital Breast Tomosynthesis Image Reconstruction // Journal of imaging. 2021. Vol. 7, N 2. P. 36. doi: 10.3390/jimaging7020036
- Cavicchioli R., Hu J.C., Loli Piccolomini E., Morotti E., Zanni L. GPU acceleration of a model-based iterative method for Digital Breast Tomosynthesis // Scientific reports. 2020. Vol. 10, N 1. P. 43. doi: 10.1038/s41598-019-56920-y
- Gomi T., Kijima Y., Kobayashi T., Koibuchi Y. Evaluation of a Generative Adversarial Network to Improve Image Quality and Reduce Radiation-Dose during Digital Breast Tomosynthesis // Diagnostics. 2022. Vol. 12, N 2. P. 495. doi: 10.3390/diagnostics1202049586.
- Cockmartin L., Bosmans H., Marshall N.W. Establishing a quality control protocol for dual-energy based contrast-enhanced digital mammography // Proceedings of the SPIE. 2021. Vol. 11595. doi: 10.1117/12.2581816
- Marimón E., Marsden P.A., Nait-Charif H., Díaz O. A semi-empirical model for scatter field reduction in digital mammography // Physics in medicine and biology. 2021. Vol. 66, N 4. doi: 10.1088/1361-6560/abd231
- Silver E.H., Shulman S.D., Rehani M.M. Innovative monochromatic x-ray source for high-quality and low-dose medical imaging // Medical Physics. 2021. Vol. 48, N 3. P. 1064–1078. doi: 10.1002/mp.14677
Дополнительные файлы