Remote monitoring of patients with chronic heart failure: A prospective randomized study
- Authors: Isaeva A.V.1, Demkina A.E.2, Vladzymyrskyy A.V.2, Zingerman B.V.3, Korobeynkova A.N.4, Bykov A.N.5, Smolenskaya O.G.1
-
Affiliations:
- Ural State Medical University
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- iPat LLC
- Center of Cardiology and Neurology
- Sverdlovsk Regional Hospital 1
- Issue: Vol 5, No 2 (2024)
- Pages: 203-218
- Section: Original Study Articles
- Submitted: 28.08.2023
- Accepted: 12.12.2023
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/568897
- DOI: https://doi.org/10.17816/DD568897
- ID: 568897
Cite item
Abstract
BACKGROUND: Chronic heart failure is one of the key problems of the Russian domestic healthcare system. E-health can be used to improve medical care quality and reduce the of hospitalizations and mortality.
AIM: To examine the effect of telemedicine monitoring on mortality, frequency of hospitalizations, and clinical and functional states of patients with chronic heart failure.
MATERIALS AND METHODS: A prospective, controlled, randomized study was conducted in the Central City Hospital No. 20 in Ekaterinburg (Russia), covering the period from December 2020 to December 2022. Patients with a confirmed diagnosis of chronic heart failure were randomized using the envelope method into three groups: group 1, a telephone control group (n=58); group 2, a remote control group using a Russian medical platform Medsenger (n=52); and group 3, the standard control group (n=103). All patients were examined, including NT-proBNP measurement and echocardiography on the first day of the study and at 3, 6, and 12 months. The occurrence of primary and secondary outcomes was evaluated at these reference points. Stata14 and jamovi software were used for statistical processing.
RESULTS: The study involved 213 participants, and all three groups were comparable in terms of basic demographic and clinical characteristics. The advantage of remote control (groups 1 and 2) over face-to-face observation in reducing cardiovascular mortality was observed after 3 (odds ratio 2.73, 95% confidence interval 1.1–7.39; p=0.042) and 12 (odds ratio 2.1, 95% confidence interval 1.1–3.7; p=0.027) months and that in reducing the occurrence of the combined primary endpoint (odds ratio 2.1, 95% confidence interval 1.1–5.6; p=0.015) after 12 months. The use of the Medsenger platform also demonstrated an advantage over face-to-face observation in the development of the combined secondary endpoint (odds ratio 1.39, 95% confidence interval 0.19–0.81; p=0.011) after 3 months and over telephone control by a nurse after 12 months in reducing cardiovascular mortality (odds ratio 0.177, 95% confidence interval 0.06–0.487; p=0.021) and development of the combined secondary endpoint (odds ratio 0.427, 95% confidence interval 0.189–0.964; p=0.041). When using the Medsenger platform, the ejection fraction increased from 47% initially to 55% after 12 months (p=0.004). The NT-proBNP level decreased from 817 to 582 pg/mL (p <0.001) after 3 months and then to 233 pg/mL after 12 months (p <0.001).
CONCLUSION: Remote monitoring protocols can be a good alternative to the traditional face-to-face monitoring of patients with chronic heart failure, which may improve clinical and functional health indicators.
Full Text
Background
Chronic heart failure (CHF) is one of the major problems of the Russian healthcare system. High mortality and readmission rates, regional demographic imbalances, poor treatment adherence, staffing shortages, and other outpatient challenges require improved management of this patient population [1, 2]. All these issues require a comprehensive approach, and eHealth can be used to address the journey of patients with CHF.
In the age of modern technology, numerous healthcare tasks are solved by systems that automate much of the work of healthcare professionals (HCPs), increasing the accessibility and quality of care at a lower cost. Patients can learn about their disease online, monitor their vital signs, communicate with their doctor, and receive medical care remotely [3, 4].
Studies of the clinical effectiveness of telemedicine monitoring have evaluated the effect of technology on all-cause and cardiovascular mortality, rate of readmission for decompensated CHF, and quality of follow-up (FU) [5]. Comparative studies of routine FU with telephone monitoring or noninvasive telemonitoring have provided valuable information on the advantages and disadvantages of these methods [6, 7]. However, the current results are highly inconsistent. The number of patients enrolled, study design, and length of FU vary widely; thus, conclusions are often quite opposite. In addition, not enough studies of high-quality design have evaluated the effect of telemedicine monitoring in the CHF population in Russia. Therefore, this study is relevant.
STUDY AIM
This study aimed to evaluate the effect of telemedicine monitoring on mortality, readmission rates, and clinical and functional status of patients with CHF.
MATERIALS AND METHODS
Study Design
This study employed an interventional, single-center, prospective, controlled, randomized, selective, unblinded design.
Eligibility Criteria
Inclusion criteria:
- Signed informed consent for outpatient CHF FU in Yekaterinburg Central City Hospital No. 20.
- Confirmed stage II–III (Strazhesko–Vasilenko classification) CHF and New York Heart Association (NYHA) functional classes (FC) I–IV.
Exclusion criteria:
- Age <18 years
- Pregnancy and lactation
- Unexplained shortness of breath
- Noncardiac edema syndrome
- Patient refusal of FU
- End-stage CHF: stage III (Strazhesko–Vasilenko classification) CHF and NYHA FC IV.
Investigational Site
Patients were enrolled in the Outpatient Heart Failure Center of Yekaterinburg Central City Hospital No. 20.
Study Duration
Patients were recruited from December 2020 to December 2021, with a post-randomization FU of 12 months.
A Medsenger electronic platform (OOO TelePat, Russia) for remote monitoring of patients with CHF was implemented after a trial period from December 1, 2020, to December 14, 2020. During this period, the system was tested on eight patients. Vital parameters such as blood pressure (BP), heart rate (HR), and body weight were evaluated in the test mode. Other elements of the system were also tested, including patient wellness data transmission, audio messaging, and video-calling options. This preparation helped in identifying some of the platform’s shortcomings in terms of CHF monitoring and developing a more convenient version of the program for all stakeholders. After the test period and following the implementation of major changes, the enrollment of patients with CHF was initiated.
Description of Medical Intervention
CHF was diagnosed according to the clinical guidelines for CHF of the Russian Society of Cardiology, which were approved by the Research and Practice Council of the Ministry of Health of the Russian Federation in 2020. The screening to confirm CHF included a medical history, general physical examination, and assessment of the FC of CHF using the 6-min walk test. All patients underwent a series of standard clinical laboratory tests (complete blood count, blood biochemistry with plasma glucose, potassium, sodium, creatinine, and calculation of the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] glomerular filtration rate), and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) measurement.
Study investigations included:
- 12-lead electrocardiography
- Echocardiography (EchoCG)
- Neck Doppler ultrasound scan
- Abdominal and pleural ultrasonography (as indicated)
- Chest X-ray imaging
After a baseline visit to a cardiologist and confirmation of a diagnosis of CHF, patients were randomized to three FU groups by the envelope method:
Group 1: Telemedicine monitoring included a structured telephone interview conducted by a nurse using a specific questionnaire (Table 2). This was done once or twice a month, depending on the baseline severity of CHF.
Table 2. Structure of remote follow-up monitoring for patients with chronic heart failure
Question | Yes | No |
Domain 1 (Patient subjective assessment) | ||
Major criteria for decompensated chronic heart failure | ||
Did you experience any shortness of breath during usual physical activity in the last week? | ||
Did you experience any shortness of breath while lying down during the week? Did you need to raise your pillow higher and/or add more pillows while sleeping? | ||
Did you experience any nighttime shortness of breath in the last week? | ||
Did you find it harder to be physically active than before in the last week? | ||
Did you feel weak, tired, or needed to rest for a long time in the last week? | ||
Did you notice swollen shins, swollen ankles, or newly appearing sock/shoe marks in the last week? | ||
Did you notice your waist getting bigger in the last week? | ||
Minor criteria for decompensated chronic heart failure | ||
Did you have night cough in the last week? | ||
Did your gain 2 kg in the last week? | ||
Did your lose 2 kg in the last week? | ||
Did you feel depressed or apathetic in the last week? | ||
Did you have heart palpitations in the last week? | ||
Domain 2 (Treatment assessment) | ||
Have you ever missed a medication dose? | ||
Do you find that you sometimes do not pay enough attention to your medication timing? | ||
Do you miss doses even when you feel well? | ||
Do you miss a dose if you feel ill after taking your medicine? | ||
Group 2: Monitoring using Medsenger telemedicine monitoring software. The methodology of telemedicine monitoring is described below.
Group 3: Control group; standard face-to-face FU with a cardiologist at a local outpatient facility in accordance with current guidelines for CHF management.
A comparative analysis was also conducted at months 3, 6, and 12. Groups 1 and 2 used remote monitoring as the main FU method (Table 1).
Table 1. Study visit procedures.
Visit Procedures | Screening and randomization (day 0) | Visit 1 (day 90 ± 30) | Visit 2 (day 180 ± 30) | Visit 3 (day 365 ± 30) |
Informed consent | + | – | – | – |
Evaluation of inclusion/exclusion criteria | + | – | – | – |
Evaluation of exclusion criteria | + | + | + | + |
Collection of medical history and previous drug therapy | + | – | – | – |
Collection of demographic and anthropometric data | + | + | + | + |
Physical examination | + | + | + | + |
Handout of a self-monitoring diary with control of its fill-out | + | + | + | + |
Treatment recommendations | + | + | + | + |
Laboratory tests and instrumental examinations | + | + | + | + |
Adherence questionnaire | + | + | + | + |
Reporting endpoints | – | + | + | + |
Assessment of satisfaction with follow-up monitoring | – | + | + | + |
In Group 1, a telephone survey and remote software monitoring were conducted by a nurse using a predesigned algorithm, with further strategies based on patient responses. Validated algorithms developed at the National Medical Research Center of Cardiology named after Academician E.I. Chazov of the Ministry of Health of the Russian Federation were used to determine the urgency of medical care [8].
In Group 2, patients received a daily notification about the need to enter their parameters (BP, HR, and body weight) into the system for clinical disease monitoring. Well-being and adherence questionnaires were sent every 3 days. In addition, all patients were instructed in advance (at the randomization visit) to perform daily self-monitoring of BP, HR, and body weight.
The remote monitoring in Groups 1 and 2 included the major and minor criteria for CHF decompensation (Table 2) based on national guidelines for CHF diagnosis and treatment.
Major CHF decompensation criteria:
- Increased shortness of breath and fatigue
- Orthopnea
- Paroxysmal nocturnal dyspnea
- Decreased exercise tolerance
- Ankle swelling
Minor CHF decompensation criteria:
- Night cough
- Weight gain >2 kg per week
- Depressive disorder
- Heart palpitations
All data obtained were processed automatically. If BP, HR, and body weight were out of range, or if the major/minor criteria for CHF decompensation were met, the program automatically sent a notification to the administrator (nurse and/or physician), who then decided on the need for a face-to-face or telemedicine consultation to change monitoring and treatment strategies. If the emergency criteria were met, an ambulance would be called. In accordance with the algorithm, the patient should receive a response to this notification within 24 h. Patients were not monitored on nonworking days, weekends, and holidays. They were instructed in advance about the need to seek medical care independently (at the local emergency room or by calling an ambulance) if their condition deteriorated. This response algorithm was used in both remote FU groups (Groups 1 and 2).
Primary Findings
The study evaluated primary and secondary endpoints.
Primary endpoints:
- Cardiovascular death
- Readmission caused by CHF decompensation
- Number of ambulance or emergency room visits caused by CHF decompensation.
Secondary endpoints:
- Death from noncardiovascular causes
- Hospital admission or emergency care visit for other cardiovascular causes (acute coronary syndrome, cerebrovascular accident, COVID-19, hypertension, etc.)
- Deterioration of the patient’s clinical and laboratory status as assessed by CHF progression: based on CHF FC, NT-proBNP levels, and left ventricular ejection fraction (LVEF).
Additional Findings
In addition, the effect of the FU option on the clinical status of a patient with CHF (NYHA FC), LVEF, and NT-proBNP levels was evaluated.
Outcome Reporting Methods
Outcomes were analyzed using medical records.
Ethical Review
The Study Protocol No. 4 dated November 25, 2020, was approved by the Local Ethics Committee of Yekaterinburg Central City Hospital No. 20. All patients provided written informed consent to participate in the study.
Statistical Analysis
Sample Size Calculation
The sample size was calculated using the method by Otdelnova KA. At a significance level of 0.05, 100 participants are sufficient for moderate precision studies [9].
Statistical Analysis
All remote and standard (face-to-face) monitoring patients were included in the data analysis. For an additional analysis, a separate remote FU group was created by pooling patients from Groups 1 and 2. Stata 14 (StataCorp, USA) and jamovi (The jamovi Project, Australia) were used in the statistical processing. Absolute values were expressed as numbers, and relative values were expressed as percentages (%). The data distribution type was determined using the Shapiro–Wilk test. Quantitative data were expressed as mean and standard deviation (M ± SD) for normal distribution and as median and interquartile range for nonnormal distribution: Me (25; 75).
Unpaired Student’s t-test and univariate analysis of variance (Mann–Whitney U and Wilcoxon tests for nonnormal distributions) were used to compare quantitative parameters in independent groups. Tukey’s post hoc analysis was performed. In the dependent groups, quantitative characteristics with a normal distribution were compared using Student’s t-test. To compare the three groups, repeated-measures analysis of variance was used. Pearson’s chi-squared test was used to compare qualitative parameters. Ordinal regression was used to compare ordinal parameters. The significance level was set at p < 0.05.
RESULTS
Study Subjects
The study enrolled a total of 213 participants. Three groups were matched for key clinical and demographic parameters, including BP, CHF duration, LVEF, estimated glomerular filtration rate (eGFR by CKD-EPI formula), hemoglobin, and NT-proBNP (Table 3). The compared groups also did not differ in the distribution of cardiovascular and noncardiac morbidity (Table 4).
Table 3. Clinical and demographic characteristics of the study groups
Parameter | Group 1 (n=58) | Group 2 (n=52) | Group 3 (n=103) | р |
Male | 36 (62.1%) | 22 (42.3%) | 42 (40.1%) | 0.143 |
Female | 22 (37.9%) | 30 (57.7%) | 61 (59.8%) | 0.265 |
Age, years | 67 (58; 72.8) | 69.5 (61.8; 79) | 69 (62; 74) | 0.159 |
BMI, kg/m2 | 32.38±5.92 | 28.71±5.46 | 32.09±6.43 | 0.236 |
SBP, mmHg | 129±22.9 | 132±16.3 | 133±17.5 | 0.305 |
DBP, mmHg | 73 (70; 90) | 80 (71; 90) | 80 (70; 90) | 0.206 |
Heart rate, bpm | 80.1±12.3 | 79.1±14.8 | 79.1±15.0 | 0.79 |
CHF duration, years | 2 (1; 5.25) | 2 (0.33; 5) | 2 (0.23; 5) | 0.167 |
LVEF, ٪ | 47 (37.8; 55) | 42 (39; 47) | 47 (39; 55) | 0.285 |
Glucose, mmol/L | 6 (5; 6) | 6.5 (6; 7) | 5 (5; 7) | 0.258 |
GFR, mL/(min × 1.73 m2) | 69 (60; 87) | 74.5 (54; 89) | 65 (55.5; 87.5) | 0.942 |
Hemoglobin, g/L | 133 (122; 146) | 133 (119; 142) | 128 (118; 141) | 0.529 |
NT-proBNP, pg/mL | 817 (331; 1480) | 617 (262; 2131) | 1082 (445; 2124) | 0.117 |
Note. BMI, body mass index; DBP, diastolic blood pressure; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure. Quality parameters are expressed as: absolute number (percentage).
Table 4. Distribution of cardiovascular and noncardiac comorbidities in the study groups
Parameter | Group 1 (n=58) | Group 2 (n=52) | Group 3 (n=103) | p |
Atrial fibrillation/atrial flutter | 32 (55,2%) | 29 (55,8%) | 62 (60,2%) | 0,467 |
Hypertension | 50 (86,2%) | 51 (98,1%) | 101 (98,1%) | 0,067 |
CHD | 32 (55,2%) | 40 (76,9%) | 83 (80,6%) | 0,087 |
Coronary revascularization | 10 (17,2%) | 7 (13,5%) | 27 (26,2%) | 0,799 |
Type 2 diabetes mellitus | 7 (12,1%) | 22 (42,3%) | 40 (38,8%) | 0,301 |
COPD | 7 (12,1%) | 13 (25,0%) | 24 (23,3%) | 0,216 |
ACE/TIA | 5 (8,6%) | 8 (15,4%) | 11 (10,7%) | 0,813 |
Note. CHD, coronary heart disease; COPD, chronic obstructive pulmonary ACE, acute cerebrovascular event; TIA, transient ischemic attack.
Primary Findings
When comparing face-to-face CHF monitoring with two remote monitoring options (pooled Groups 1 and 2), a benefit of remote monitoring in reducing cardiovascular mortality was observed at 3 months with an odds ratio (OR) of 2.73 (95% confidence interval [CI], 1.1–7.39, p = 0.042). No benefit was seen in terms of the effect on the composite secondary endpoint (all-cause mortality, hospital admission, or emergency visits for other cardiovascular causes, deterioration of the patient’s clinical and laboratory status) at 3 months: OR 0.5; 95% CI 0.27–0.92; p = 0.03 (Fig. 1).
Fig. 1. Effect of outpatient follow-up option (remote/face-to-face) on month 3 endpoints. CHF, chronic heart failure.
When comparing groups 1 and 2 of remote FU in terms of the effect on month 3 endpoints, the Medsenger platform demonstrated an advantage over face-to-face monitoring in the composite secondary endpoint: OR 0.39; 95% CI, 0.19–0.81; p = 0.011.
After 6 months of FU, no significant differences were found between distance and face-to-face FU (Fig. 2). However, the number of readmissions due to acute CHF decompensation was significantly higher in the face-to-face FU group than in the Medsenger group: OR 0.171; 95% CI: 0.04 to 0.83; p = 0.029.
Fig. 2. Effect of outpatient follow-up option (remote/face-to-face) on month 6 endpoints. CHF, chronic heart failure.
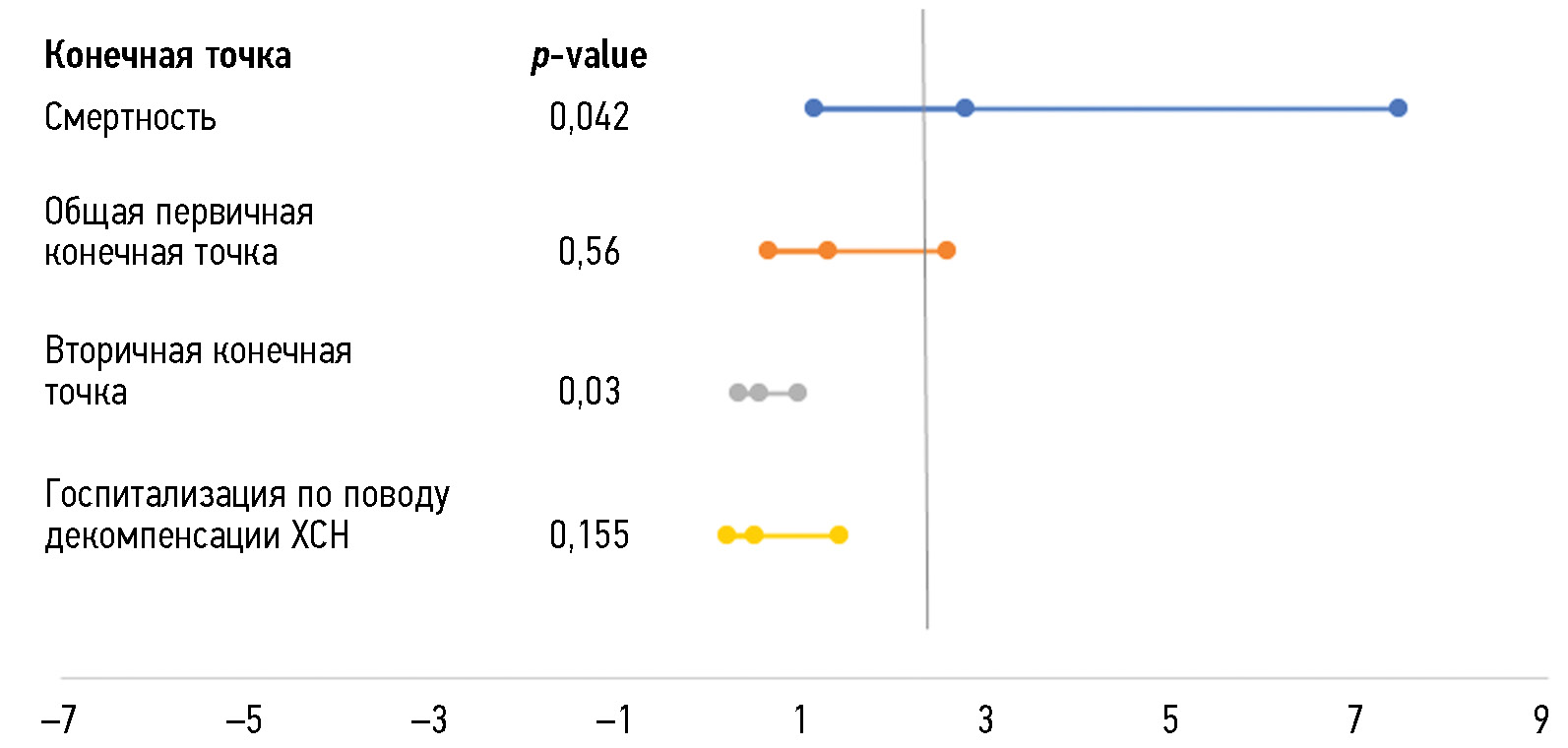
After 12 months of FU, the remote monitoring options demonstrated superiority over face-to-face FU for patients with CHF in terms of reducing cardiovascular mortality (OR 2.1; 95% CI, 1.1–3.7; p = 0.027) and the combined primary endpoint (OR 2.1, 95% CI 1.1–5.6; p = 0.015) (Fig. 3).
Fig. 3. Effect of outpatient follow-up option (remote/face-to-face) on month 6 endpoints. CHF, chronic heart failure.
However, when comparing the remote monitoring options, the Medsenger platform was found superior to a structured telephone survey conducted by a nurse at 12 months of FU for cardiovascular mortality (OR 0.177, 95% CI 0.06–0.487; p = 0.021) and a composite secondary endpoint (OR 0.427, 95% CI 0.189–0.964; p = 0.041).
Additional Findings
In the remote FU group (Groups 1 and 2), LVEF increased significantly by 6%–7%, from 43% (38–54) at the randomization visit to 49% (41–56) at the month 3 visit (p < 0.001) and up to 50% at the month 6 visit (p = 0.045). However, no further increase in LVEF (at month 6 and 12 visits) was reported in the remote FU group.
No significant increase in LVEF was found in the face-to-face FU group (Group 3). During the period from the randomization visit to month 3 FU visit, LVEF increased by 4%, from 47% (39–55) to 51% (42–60), and then decreased to 48% (38.8–55.3) at month 6 visit (p = 0.475).
In the remote FU group using the Medsenger platform, LVEF increased significantly from 47% (37.8–55) at baseline to 55% (41.3–60) at the end of FU (p = 0.004) (Fig. 4).
Fig. 4. Changes in the left ventricular ejection fraction in the study groups. Group 1, telephone follow-up (n = 58); Group 2, Medsenger follow-up (n = 52); Group 3, standard follow-up (n = 103).
In the remote FU group (pooled groups 1 and 2), NT-proBNP levels decreased both in the short term (at month 3, from 717 [296.5–1805.5] pg/mL to 464.4 [181.5–861] pg/mL, p < 0.001) and during the entire FU period (from 717 [296.5–1805.5] pg/ mL to 294.5 [133.5–817] pg/mL, p < 0.001). In the face-to-face group, a positive trend was reported for this marker; however, only at months 3 and 6 (p < 0.001) (Fig. 5).
Fig. 5. Changes in NT-proBNP levels by follow-up options; remote follow-up: pooled group (groups 1 and 2, n = 110); face-to-face follow-up: standard follow-up group (n = 103).
When evaluating the effect of the different FU options on changes in NT-proBNP levels in the study groups, the greatest decrease in this parameter at months 3 and 6 was found in the structured telephone survey group: from 617 (262–2,131) pg/mL to 345 (321–923) pg/mL at month 6 (p = 0.007). No difference was reported over 12 months. The Medsenger medical platform was superior at months 3 and 6: at 3 months, the NT-proBNP levels decreased from 817 (331–1,480) pg/mL to 582 (208–896) pg/mL (p < 0.001), and at 1 year, it decreased by 3.5 times to 233 (128–638) pg/mL (p < 0.001) (Fig. 6).
Fig. 6. Changes in NT-proBNP levels in the study groups; Group 1, telephone follow-up (n = 58); Group 2, Medsenger follow-up (n = 52); Group 3, standard follow-up (n = 103).
From a technical perspective, assessing the effect of FC on outcomes and determining the effectiveness of the monitoring options are challenging owing to the ordinal nature of the variable, missing data (patient deaths), and presence of related groups (groups were assessed at different time points). Therefore, a method was chosen to assess the effect of the FC on the outcomes in each FU group by using ordinal regression.
In Group 1, no significant influence of FC on the outcomes was found by telephone interviews. Group 2 using the Medsenger platform showed that at 3 months, the FC level had a significant effect on the secondary endpoint (p = 0.016) and the admission rate due to CHF decompensation (p = 0.006). Similar results were reported at 12 months (p < 0.001), and no difference was seen at 6 months. In Group 3, standard FU showed that the FC affected the primary endpoint only after 12 months (p = 0.021), without affecting other outcomes. No significant effects were found when comparing the remote and face-to-face FU groups.
Adverse Events
No adverse events were reported for the study technology.
Discussion
Summary of Primary Findings
The study demonstrated the superiority of remote FU over routine monitoring in patients with CHF in reducing cardiovascular mortality at 3 and 12 months of FU. In the remote FU group, LVEF increased by a significant 6% during the first 3 months, and NT-proBNP levels decreased at all reference points. The Medsenger platform was superior to a telephone survey at 12 months of FU for cardiovascular mortality and a composite secondary endpoint. It was also associated with an increase in LVEF (from 47% at baseline to 55% at the end of FU) and a decrease in NT-proBNP levels (from 817 pg/mL to 233 pg/mL).
Discussion of Primary Findings
To date, many clinical studies have evaluated various telemedicine technologies, including telemedicine monitoring for chronic noncommunicable diseases [10]. However, even today, no clear understanding of its clinical effectiveness has been reached in terms of its effect on cardiovascular mortality and readmissions. As a result, the implementation of telemedicine technologies in the routine practice for the long-term care of patients with chronic noncommunicable diseases is limited.
In the multicenter European iCOR study, remote monitoring of the health status of patients with CHF using videoconferencing and telemetry of biological parameters was associated with a significant reduction in the rates of acute LV failure from 56% to 22% and a decrease in the treatment cost from €8,163 to €4,993 over 6 months [11].
The first Cochrane review of the effectiveness of CHF telephone support and telemonitoring was published in 2007 and updated in 2010. The latest version of the review included 30 randomized clinical studies with 9,806 patients. The primary outcome of this meta-analysis was that patients followed up using telemonitoring systems had lower all-cause mortality (relative risk [RR] 0.66, 95% CI 0.54–0.81; p < 0.001) and heart failure (HF) admissions (RR 0.79, 95% CI 0.67–0.94, p = 0.008), whereas telephone patient support affected only on HF admissions (RR 0.77, 95% CI 0.68–0.87; p < 0.0001) [12].
Some studies have shown that remote monitoring is effective in reducing all-cause mortality, CHF admission, and mortality in this patient population [13, 14].
OSICAT was a multicenter, randomized study (n = 937) that enrolled patients with decompensated HF within ≤1 year before the study started. Remote monitoring was associated with a 21% decrease in the relative risk of first emergency admission (OR 0.79, 95% CI 0.62–0.99; p = 0.044), a 29% decrease in the relative risk in patients with NYHA FC III or IV HF (OR 0.71, 95% CI 0.53–0.95; p = 0.02), and a 38% relative risk decrease in socially isolated patients (OR 0.62, 95% CI 0.39–0.98; p = 0.043) [15].
In subsequent years, the publication of several large randomized clinical studies with negative [16–18] and neutral [19, 20] results led to increasing uncertainty about the benefits of telephone support and telemonitoring.
A systematic review and meta-analysis of studies enrolling patients with recently decompensated HF revealed that remote FU was not superior to standard FU in terms of all-cause admission (OR 0.95, 95% CI 0.84–1.08, p = 0.43) and all-cause mortality (OR 0.83, 95% CI 0.63–1.09, p = 0.17) rates [21].
A systematic review of 34 randomized clinical studies (2000–2021) found that remote monitoring had no significant effect on reducing hospital admissions or mortality. American studies in patients with CHF and a high risk of readmission showed positive trends in reducing mortality/admission rates; however, no significant differences were reported [22].
Notably, these studies had several limitations, such as recruitment of patients with severe HF with LVEF <35%, differences in treatment adherence between groups, low study power, short duration of patient FU, and lack of uniform standards for telemedicine monitoring. All these factors prevent a comprehensive assessment of the effectiveness of FU options.
This study showed a clear association between the effect of the remote monitoring option and the primary endpoint, remote monitoring resulted in 2.73-fold decrease in mortality rate, and these differences persisted throughout the study. When comparing the remote FU options in the short term, no differences were found; however, at 12 months, Medsenger monitoring was superior to structured telephone support with a 17% decrease in cardiovascular mortality (p = 0.029). Such results may be related to the ability of a physician to adjust therapy remotely through telemedicine consultation, which may prevent further HF decompensation and death. In addition, educating patients on what to do if their condition worsens also helps prevent adverse outcomes [23].
The decreased number of deaths and admissions in the remote monitoring group is explained by the improvement in the clinical and functional profiles of patients. Our data show that this monitoring option is associated with an increase in LVEF and a decrease in NT-proBNP levels, indicating CHF stabilization and improvement in the quality of life. Patients in the remote monitoring groups received regular support from HCPs, and this had a positive effect on adherence.
Literature data on the effect of the FU option on laboratory tests and investigations are limited, probably due to the high cost of these types of diagnostics. Single studies evaluated the effect of the FU option on FC in patients with CHF. For example, Pyrikova NV et al. [25] demonstrated a positive effect of remote FU on the functional status of patients with CHF. At 6 months, the number of patients with FC III decreased by 1.7 times (p = 0.03), and in the control group with routine monitoring, it increased by 1.9 times (p = 0.002). FC IV was 6.5 times more frequent in the control group than in the telemedicine groups, indirectly indicating the effectiveness of the latter. The present study reported the superiority of Group 2 monitoring: when using the Medsenger platform, the FC level showed a significant effect on the secondary endpoint and admission rate due to decompensated CHF at 3 months (p = 0.006). This may also indicate that the improved clinical status of patients in this group positively affected the outcome. In the standard monitoring group, FC affected the primary endpoint only at 12 months, whereas no association was found in the telephone survey group.
Thus, remote FU positively affects LVEF, NT-proBNP levels, and FC. The normalization of these clinical and functional parameters is likely to decrease the cardiovascular mortality rate and the incidence of composite secondary endpoint events and explains the positive results in remote FU.
Study Limitations
The sample size was calculated using the moderate precision test and a significance level of 0.05. To increase the power of the study and the representativeness of the results with a similar design, more participants would be required.
Conclusion
One of the most important and challenging issues in modern healthcare is finding a way to best monitor patients with CHF to improve outcomes and reduce mortality and admission rates. Remote monitoring protocol based on our data can become a good alternative to traditional face-to-face monitoring. The results showed the superiority of remote monitoring to standard monitoring, with decreased cardiovascular mortality rates at 3 and 12 months (OR 2.73, 95% CI 1.1–7.39, p = 0.042, and OR 2.1, 95% CI 1.1–3.7, p = 0.027, respectively).
Improving the clinical and functional status of patients will also improve patient outcomes, their quality of life and level of self-care, contribute to adherence and trust in the healthcare system, and drive the development of related IT technologies. The widespread adoption of such solutions is currently limited by barriers such as low patient digital literacy, a lack of clear attitudes toward remote monitoring in the healthcare community, and need for additional technological equipment in medical facilities. However, the demographic characteristics of our population, growing number of patients with CHF, and need for high-quality, personalized care will ultimately drive the universal adoption of remote monitoring technologies.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. A.V. Isaeva — the concept of the study, data collection, writing the text of the manuscript; A.E. Demkina — the concept of the study, writing the text of the manuscript; A.V. Vladzymyrskyy — the concept of the study, manuscript text editing; B.V. Zingerman — scientific project management; A.N. Korobeynikova — data analysis, statistics analysis, writing the text of the manuscript; A.N. Bykov, O.G. Smolenskaya — data analysis, manuscript text editing.
About the authors
Anna V. Isaeva
Ural State Medical University
Email: av_isaeva_cgb20@mail.ru
ORCID iD: 0000-0003-0634-9759
SPIN-code: 5178-6596
MD, Cand. Sci. (Medicine)
Russian Federation, EkaterinburgAlexandra E. Demkina
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: ademkina@bk.ru
ORCID iD: 0000-0001-8004-9725
SPIN-code: 4657-5501
MD, Cand. Sci (Medicine)
Russian Federation, MoscowAnton V. Vladzymyrskyy
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: a.vladzimirskiy@npcmr.ru
ORCID iD: 0000-0002-2990-7736
SPIN-code: 3602-7120
MD, Dr. Sci. (Medicine)
Russian Federation, MoscowBoris V. Zingerman
iPat LLC
Email: boriszing@gmail.com
ORCID iD: 0000-0002-1855-1834
SPIN-code: 5914-9174
Russian Federation, Moscow
Anna N. Korobeynkova
Center of Cardiology and Neurology
Author for correspondence.
Email: anna_best2004@mail.ru
ORCID iD: 0000-0002-8934-7021
SPIN-code: 9728-9583
MD, Cand. Sci. (Medicine)
Russian Federation, KirovAlexandr N. Bykov
Sverdlovsk Regional Hospital 1
Email: sashacor83@yandex.ru
ORCID iD: 0000-0003-0787-7908
SPIN-code: 6423-7610
MD, Cand. Sci. (Medicine)
Russian Federation, EkaterinburgOlga G. Smolenskaya
Ural State Medical University
Email: osmolenskaya@mail.ru
ORCID iD: 0000-0002-0705-6651
SPIN-code: 5443-9382
Dr. Sci. (Medicine), Professor
Russian Federation, EkaterinburgReferences
- Tereshchenko SN, Galyavich AS, Uskach TM, et al. Clinical practice guidelines for Chronic heart failure. Russian Journal of Cardiology. 2020;25(11):40–83. EDN: LJGGQV doi: 10.15829/1560-4071-2020-4083
- Mareev VYu, Fomin IV, Ageev FT, et al. Guidelines for Heart failure: chronic (CHF) and acute decompensated (ADHF). Diagnosis, prevention and treatment. Kardiologiia. 2018;58(6S):8–158. EDN: XUAREL doi: 10.18087/cardio.2475
- Vladzimirskii AV, Morozov SP, Urvantseva IA, Kovalenko LV, Vorob›ev AS. Application of telemedicine technologies in cardiology. Surgut: Surgut State University; 2019. (In Russ). EDN: IKCWRK
- Vladzimirskii AV, Lebedev GS. Telemedicine. Moscow: GEOTAR-Media; 2018. (In Russ). EDN: YMURZR
- Vladzimirskii AV. Telemedicine in cardiology: opportunities and evidence. Zamestitel’ glavnogo vracha. 2016;(8):80–89. (In Russ). EDN: WFLUGF
- Veenis JF, Radhoe SP, Hooijmans P, Brugts JJ. Remote Monitoring in Chronic Heart Failure Patients: Is Non-Invasive Remote Monitoring the Way to Go? Sensors (Basel). 2021;21(3):887. doi: 10.3390/s21030887
- Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JGF. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;(10). doi: 10.1002/14651858.CD007228.pub3
- Boytsov SA, Ageev FT, Blankova ZN, Svirida ON, Begrambekova YuL. Guidelines for nurses and patients with chronic heart failure. Cardiovascular Therapy and Prevention. 2021;20(1):283–306. EDN: WNIVZB doi: 10.15829/1728-8800-2021-2754
- Bavrina AP. Basic concepts of statistics. Meditsinskii al’manakh. 2020;(3):101–111. EDN: PUMGMM
- Arzamasov KM, Bushuev VO, Vladzymyrskyy AV, et al. Examination of a cardiac patient, by using carotid artery ultrasound screening: possibilities of telemedicine. Vrach. 2023;34(4):39–45. EDN: LRZKEW doi: 10.29296/25877305-2023-04-08
- Jiménez-Marrero S, Yun S, Cainzos-Achirica M, et al. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: A sub-analysis of the iCOR randomized trial. J Telemed Telecare. 2020;26(1-2):64–72. doi: 10.1177/1357633X18796439
- Clark RA, Inglis SC, McAlister FA, Cleland JGF, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334(7600):942. doi: 10.1136/bmj.39156.536968.55
- Mizukawa M, Moriyama M, Yamamoto H, et al. Nurse-led collaborative management using telemonitoring improves quality of life and prevention of rehospitalization in patients with heart failure. Int Heart J. 2019;60(6):1293–1302. doi: 10.1536/ihj.19-313
- Dierckx R, Inglis SC, Clark RA, Prieto-Merino D, Clelandet JGF. Telemedicine in heart failure: new insights from the Cochrane meta-analyses. Eur J Heart Fail. 2017;19(3):304–306. doi: 10.1002/ejhf.759
- Jiménez-Marrero S, Yun S, Cainzos-Achirica M, et al. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: A sub-analysis of the iCOR randomized trial. J Telemed Telecare. 2020;26(1-2):64–72. doi: 10.1177/1357633X18796439
- Galinier M, Roubille F, Berdague P, et al. OSICAT Investigators. Telemonitoring versus standard care in heart failure: a randomised multicentre trial. Eur J Heart Fail. 2020;22(6):985–994. doi: 10.1002/ejhf.1906
- Lynga P, Persson H, Hägg-Martinell A, et al. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail. 2012;14(4):438–444. doi: 10.1093/eurjhf/hfs023
- Boyne JJ, Vrijhoef HJ, Crijns HJ, et al. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012;14(7):791–801. doi: 10.1093/eurjhf/hfs058
- Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473
- Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99(23):1717–1726. doi: 10.1136/heartjnl-2013-303811
- Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029
- Umeh CA, Reddy M, Dubey A, et al. Home telemonitoring in heart failure patients and the effect of study design on outcome: A literature review. J Telemed Telecare. 2021;30(1). doi: 10.1177/1357633X211037197
- Drews TEI, Laukkanen J, Nieminen T. Non-invasive home telemonitoring in patients with decompensated heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(5):3696–3708. doi: 10.1002/ehf2.13475
- Vladzymyrskyy AV. Systematic review: the messengers “WhatsApp®” and “Viber®” in a clinical routine. Zhurnal telemeditsiny i elektronnogo zdravookhraneniya. 2017;1(3):30–41 EDN: YPTUYR
- Pyrikova NV, Mozgunov NA, Osipova IV. Results of pilot remote monitoring of heart failure patients. Cardiovascular Therapy and Prevention. 2022;21(6):31–51. EDN: ROTHHY doi: 10.15829/1728-8800-2022-3151
Supplementary files