Multiparametric magnetic resonance imaging and magnetic resonance imaging fusion-guided biopsy for the diagnosis of prostate cancer: current status
- Авторлар: Testini V.1,2, Eusebi L.3, Guerra F.S.1, Giannubilo W.4, Di Biase M.5, Russo A.2, Guglielmi G.1,2,6
-
Мекемелер:
- Foggia University
- Monsignor Raffaele Dimiccoli
- Carlo Urbani Hospital
- Ospedale Civile
- Santa Maria della Misericordia Hospital
- Casa Sollievo della Sofferenza Hospital
- Шығарылым: Том 5, № 2 (2024)
- Беттер: 283-302
- Бөлім: Reviews
- ##submission.dateSubmitted##: 10.11.2023
- ##submission.dateAccepted##: 27.12.2023
- ##submission.datePublished##: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/623183
- DOI: https://doi.org/10.17816/DD623183
- ID: 623183
Дәйексөз келтіру
Аннотация
This review explains the role of multiparametric magnetic resonance imaging, particularly in prostate biopsy, in the detection of prostate cancer. The use of multiparametric magnetic resonance imaging in the diagnosis of prostate cancer has also allowed its use in magnetic resonance imaging-guided biopsies, which according to many studies present high sensitivity and specificity in early diagnosis and staging, in patients with persistently high prostate-specific antigen levels despite previous negative prostate biopsies, and in the follow-up of patients under active surveillance.
To perform a targeted prostate biopsy, three types of magnetic resonance imaging guidance are available: cognitive fusion, direct magnetic resonance imaging-guided biopsy performed within a tomograph (in-bore biopsies), and software coregistration of stored magnetic resonance images with real-time ultrasound using a fusion device, with multiparametric magnetic resonance imaging findings digitally overlaid on real-time transrectal ultrasound images for targeted biopsy.
Each method has its advantages and disadvantages. Magnetic resonance imaging-targeted biopsy improves the quality of histological results compared with other approaches, with approximately 90% correct detection of significant index lesions. Correct staging allows the selection of the best therapeutic options, adequate evaluation of the prognosis, and reduction of the incidence of new biopsies and complications. The current objective is to make magnetic resonance imaging-guided biopsy increasingly available and standardize the technique to minimize inter-operator variability depending on the available system.
Толық мәтін
INTRODUCTION
In Western countries, prostate cancer (PCa) is the most frequent noncutaneous tumor in men [1]. According to the European Association of Urology (EAU) recommendations, prostate biopsy is the gold standard for PCa diagnosis.
The first diagnostic biopsy method is the random technique (rather than sextant), with 12 samples taken only in the peripheral zone [2]. This technique has limitations such as undersampling (insufficient number of samples in relation to the prostate volume), oversampling (excessive core number, with identification of the nonsignificant microfoci), overstaging, with a diagnosis of nonsignificant tumors (low sensitivity), understaging, with failure to diagnose clinically significant cancers (low specificity), and operator experience-related errors [3].
The new therapeutic trend of active surveillance (AS; for low-risk tumors and minimally invasive targeted procedures necessitates more precision in prostate examination, necessitating advancements in the biopsy approach.
Prostate biopsy is currently recommended for men aged 50–69 years with high serum prostate-specific antigen (PSA) levels (>3 ng/mL) or abnormal digital rectal examination findings (nodules, induration, and asymmetry). After the first set of negative biopsy results, when suspicion of PCa remains high, the number of samples (i.e., saturation biopsy) and sampling of the transitional zone should be increased [4].
Since its initial application in 1983, MRI has been widely used for PCa diagnosis because of its increased availability and multiparametric imaging, which combines anatomical and functional data [5, 6].
In 2012, the European Society of Urogenital Radiology published guidelines based on expert consensus to standardize the evaluation and reporting of prostate MRI: the Prostate Imaging Reporting and Data System (Pirads) [7]. Since then, the Pirads score has been externally evaluated and found to be reliable for more accurate PCa detection [8].
In 2019, the Pirads Steering Committee published updated reporting guidelines describing the assessment categories and technical parameters described in version 2 of the Pirads [9].
With these premises, the role of multiparametric MRI (mpMRI) has become central not only in the diagnosis of PCa but also in biopsy as a tool for correct staging and assessment of tumor extension (Gleason score [GS]).
The current goal is to make MR-guided biopsy increasingly available, thereby minimizing interoperator variability depending on the available system.
METHODS OF MAGNETIC RESONANCE IMAGING GUIDED PROSTATE BIOPSY
To perform a targeted prostate biopsy, three types of MRI guidance are available:
- Cognitive fusion, in which the operator visually directs the transrectal ultrasound (TRUS)-guided biopsy to the prostate area of abnormalities on mpMRI.
- Direct MRI-guided biopsy, performed within an MRI tube (in-bore biopsies): This technique is quite precise for identifying areas of interest within the prostate; however, it is time-consuming, expensive, and impractical because the entire process is performed within the MRI gantry [10].
- Software coregistration of stored MRI with real-time US using a fusion device, with mpMRI findings digitally overlaid on real-time TRUS images for targeted biopsy (TB). This procedure can be performed using elastic fusion systems (g., Koelis Urostation and Eigen Artemis) or rigid fusion systems (e.g., Philips Uronav, Eindhoven, The Netherlands; Medcom BiopSee, Darmstadt, Germany).
Each method has its advantages and disadvantages.
Despite substantial variation in the sensitivity of detecting clinically indolent disease across studies, most studies have shown that the sensitivity of MR–US fusion (cognitive or device) ranges between 80% and 95% [11].
The main clinical indications for MR–US fusion biopsy are persistently high PSA levels despite previous negative prostate biopsies and follow-up of patients on AS [12].
Cognitive Fusion
Cognitive fusion is performed easily and rapidly and does not require any additional equipment other than an MRI and a standard TRUS facility. The US operator does not need any additional training beyond the standard TRUS-guided biopsy. Cognitive fusion is based on the sonographer’s ability to localize the region of interest by imaging its location within the prostate after viewing the worrisome lesion on MRI [12].
MRI and TRUS images are superimposed by a cognitive overlay during biopsy, which can be performed with a printed document or by presenting MR images on the screen of a workstation in the TRUS room adjacent to the TRUS platform [13].
The physician aims at the target lesion with knowledge of lesion localization on MRI. The advantages of cognitive fusion are speed and simplicity; that is, it does not require additional equipment beyond what is ordinarily necessary for a TRUS-guided biopsy [14].
However, one of the downsides of cognitive fusion biopsy approaches is the possibility of sampling error when attempting to conceptualize the region of interest, which is particularly problematic for smaller tumors. Another disadvantage is the inability to track the position of each previous biopsy.
Despite this, the tumor detection rate of cognitive fusion is comparable to that of device-mediated fusion, and in most investigations, both cognitive and device-mediated fusions appear to be superior to random systematic sampling alone [15, 16].
The disadvantage of cognitive fusion is the possibility of human error in extrapolating from MRI to TRUS in the absence of a real overlay.
Several studies have assessed the value of cognitively performed TB. Lawrentschuk et al. found that cognitive TB performed better than random cores, particularly in anterior lesions [17]. In a retrospective analysis, Haffner et al. compared TB results with those of 12 random biopsies in 555 patients [18]. A TB approach alone would have required just 3.8 cores per patient, thereby avoiding unnecessary biopsies in 38% of patients with normal MRI findings and avoiding the detection of minor cancer discovered by random biopsies in 13% of cases [18].
In this study, 13 significant cancers were missed with TB alone, whereas 12 significant cancers were missed with the standard approach [18].
In another study, Puech et al. discovered that MRI before biopsy increased the cancer detection rate (CDR) from 59% by 12-core saturation biopsy (SB) to 65% by cognitive TB [15].
In terms of significant cancer (cancer core length > 3 mm on any core and GS > 3 + 3), the CDRs were 67% for TB and 52% for conventional biopsies [15].
Labanaris et al. demonstrated that TB allowed for a 90% match between biopsy and surgical GS and indicated that MRI should be performed before biopsy to decrease SB underestimation of GS [19].
Thus, current research suggests that MRI-guided biopsy has higher accuracy and CDRs than standard TRUS-guided biopsy.
Haffner et al. reported that cognitive fusion is no better than systematic biopsies in men with questionable MRIs, highlighting the major risk associated with cognitive fusion: interobserver variability [18]. Although the present literature on cognitive fusion shows potential in the hands of professional cognitive fusionists, most urologists are now shifting toward commercially accessible device-mediated platforms because of the lack of tracking and digital overlay.
Direct Magnetic Resonance Imaging Guided Biopsy is Performed ‘In-Bore’
A radiologist performs direct MRI-guided biopsy “in-bore,” that is, within the MRI tube, by fusing a previous MR image indicating a lesion with a contemporaneous MR image to confirm biopsy needle localization. The transrectal route is employed. After each biopsy sample, the patient is scanned to ensure localization. Typically, only a few targeted cores are taken, and systematic sampling is not conducted.
The in-bore biopsy method provides the benefits of precision needle placement, fewer sampled cores, and a low risk of missed targets if they are visible by MRI [20]. Its disadvantages include greater expense and time consumption and the inability to regularly sample the residual gland [20]. This is relevant because MRI misses approximately 10% of severe lesions compared with final RP pathology [21, 22].
Quentin et al. showed that in-bore TB has an excellent significant CDR of 92.2% [23]. Hoeks et al. reported that 265 patients with dubious lesions on mpMRI who had previously negative TRUS biopsies had transrectal in-bore TB, resulting in a CDR of 41%, with 87% of the identified tumors being clinically significant [24].
The Barentsz group at Radboud University in Nijmegen, The Netherlands, presented a vast experience with in-bore biopsy [25]. The benefits of this procedure include a reduced number of cores taken, precise localization of the biopsy, and less detection of insignificant tumors. The downsides of this procedure include the time and cost, including the in-bore time, and two MRI sessions are required to obtain the biopsy specimens.
Furthermore, because only suspicious lesions are sampled, tissues with a “normal” MRI appearance are not collected, which is problematic because any false-negative characteristics of prostate MRI are unknown [25].
Fusion of Magnetic Resonance Imaging and Transrectal Ultrasound
In this method, the operator images the prostate using US, as has been used for decades. While viewing the prostate, the MR images of that prostate, in which imaging was performed beforehand and images were stored in the device, are fused with real-time US images using a digital overlay, allowing the target(s) previously delineated by a radiologist to be brought into the US machine’s aiming mechanism. Fusion produces a three-dimensional (3D) reconstruction of the prostate, and the aiming and tracking of biopsy sites occur on the reconstructed model (Fig. 1 and 2) [25]. Several commercial platforms are now available, each with a different method of coregistration and a different hardware platform for lining up the biopsy with the coregistered picture [3]. Compared with VE, MRI–TRUS fusion-guided biopsy may have higher reproducibility because of less operator dependence and delivery of real-time feedback on actual biopsied areas [3]. The disadvantages include higher software/device costs, dependence on software for accuracy, and the associated learning curve and operator training [26] (Fig. 3 and 4).
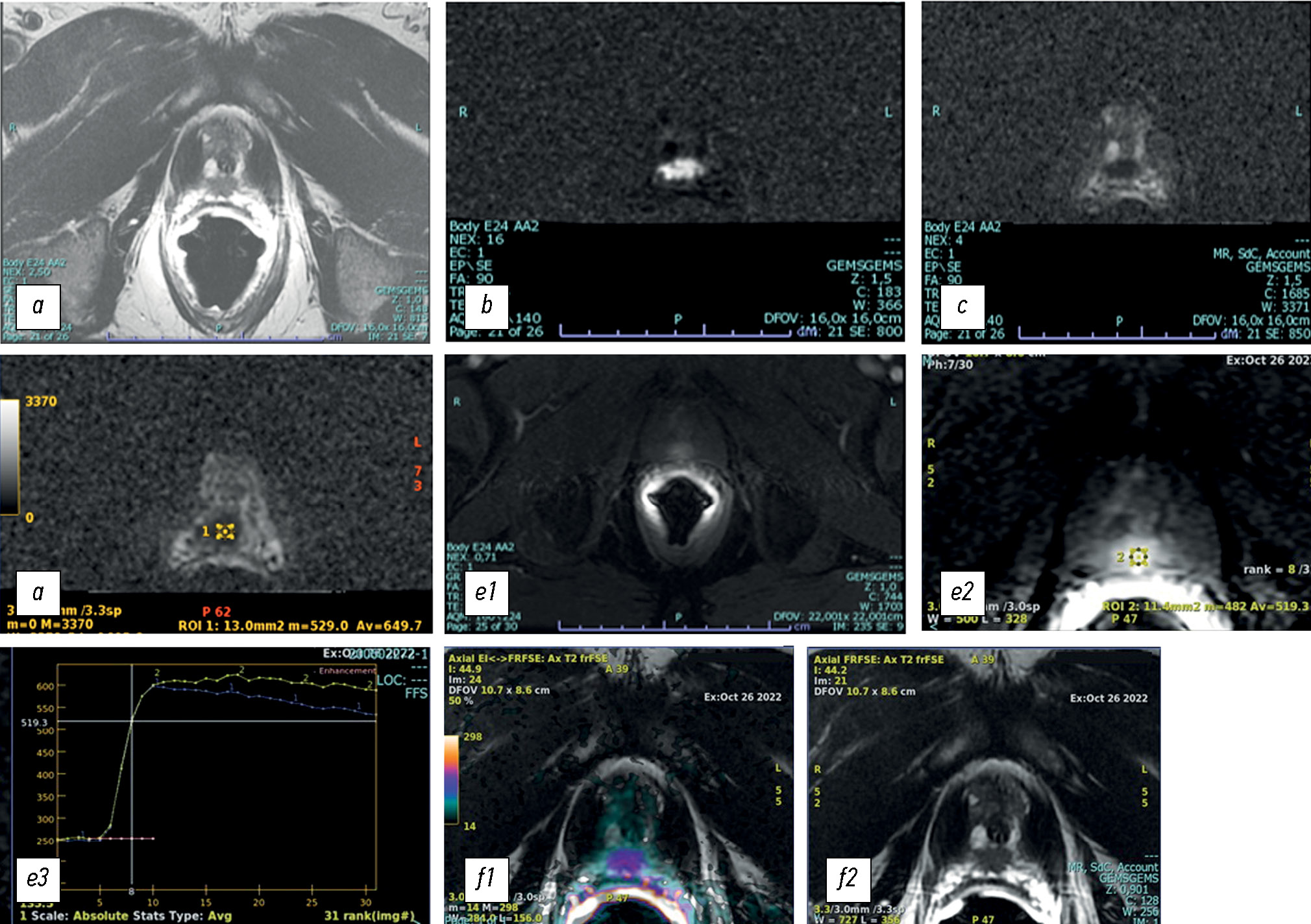
Fig. 1. T2-weighted (a) magnetic resonance image (axial plane) showing a hypointense nodular lesion in the peripheral zone at the apical posterior site. Axial diffusion-weighted MR image (b) with an apparent diffusion coefficient (ADC) (c) of put value × 10−3 m2/sec in the corresponding area. Reduced water diffusion in prostate cancer (PCa) is related to increased cellularity of malignant lesions, with a reduction in the extracellular space and restriction of movement of a larger portion of water molecules into the intracellular space. Therefore, DWI with the corresponding ADC map provides an important quantitative biophysical parameter that can be used to diagnose PCa (d). The tumor is characterized by intense early hyperenhancement of the normal adjacent tissue (e). Colorimetric map generated by the DCE evaluation of PCa in the peripheral area at the apical site. The suspicious area is coded in red (f).
Fig. 2. MRI–TRUS fusion-guided prostate biopsy. The prostate and lesions identified on MR images (in this case, on T2W imaging) are segmented. During the procedure, a 2D TRUS scan of the prostate was performed. Images were recorded semiautomatically. Co-viewing images can be tiled or overlaid. By merging the two images, the urologist can locate the lesion during a biopsy using real-time ultrasound guidance.
Fig. 3. Magnetic resonance (MR) imaging of prostate cancer: axial diffusion-weighted MR image (up on the left) with an apparent diffusion coefficient map (down on the left), T2-weighted MRI image (up on the right), and T1-weighted image after contrast media (down on the right).
Fig. 4. Magnetic resonance imaging is fused with real-time TRUS using a digital overlay, allowing the target(s) previously delineated by a radiologist to be brought into the ultrasound machine’s aiming mechanism. Fusion enables the reconstruction of the prostate, and the aiming and tracking of biopsy sites occur on the reconstructed model.
This method has the disadvantage of being an indirect method, requiring the use of an additional device, and requiring specialized operator training. The benefit is that it may be performed in minutes in an outpatient clinic environment under local anesthesia, using techniques that have been used for years. The results obtained using the fusion device are highly encouraging [25].
Device-mediated fusion allows for the targeting of biopsies into previously defined MRI regions of interest using a 3D rendering apparatus that superimposes saved MR images on real-time US images, a technique known as coregistration. Although several commercial MR–US fusion platforms are available, all use some types of image coregistration and needle/probe tracking (mechanical or electromagnetic). These technologies enable the acquisition, storage, and reconstruction of real-time US images and the creation of 3D maps of lesion locations and prior biopsy sites for future reference [27]. Because any movement of the patient or the prostate affects image coregistration, these fusion devices also employ dynamic repeat registration methods using motion-compensation algorithms to guarantee accurate and reproducible index lesion targeting [12].
Recent studies have compared the detection of PCa and severe disease using traditional SB or TPMB as a reference test [22, 28, 29].
In a cohort of >1000 patients, Siddiqui et al. used the Uronav system and conventional 12-core TRUS-guided biopsy as a reference test and reported that TB diagnosed 30% more high-risk cancers than standard biopsy (P = 0.001) and 17% fewer low-risk cancers (P = 0.002) using primary GS 4 as the significance level [22]. Rastinehad et al. defined GS 3 + 4 as clinically significant PCa (sPCa) and reported that 14.3%–20.9% of sPCa cases were discovered by TB alone but ignored by the traditional TRUS technique [30]. Furthermore, 23.5% of cases were upgraded from insignificant to sPCa through MRI–TRUS fusion-guided biopsy [30]. In contrast, 4/105 sPCa cases were missed by MRI–TRUS fusion-guided biopsy [30].
Baco et al. demonstrated that 98% of index tumors defined as the highest GS or biggest volume in the case of multifocality with equal GS, were diagnosed by MRI and that 98% of the correct location was diagnosed by MRI–TRUS fusion-guided TB using the Koelis Urostation® [31].
Regarding public health, Cerantola et al. published a cost-effectiveness analysis comparing TRUS biopsy with MRI target biopsy (MRTB) in 2016. They assessed the cumulative effects after 5, 10, 15, and 20 years and determined that including MRI and MRTB in PCa diagnosis and care is a cost-effective measure after 5, 10, 15, and 20 years [32].
COMPARATIVE STUDIES OF DIFFERENT TARGETED BIOPSY APPROACHES
Only a few studies have examined the CDRs of various targeting strategies, and the results are contradictory [26].
Delongchamps et al. reported that cognitive fusion biopsy was not significantly better than SB in a study comparing VE with two MRI–TRUS fusion devices; however, both software coregistration devices tested (Esaote/MyLabTMTwice and Koelis/Urostation) significantly increased the CDRs compared with SB in a cohort of 391 patients using conditional logistic regression analysis [33].
In a prospective trial of 125 men with worrisome lesions, Wysock et al. compared MRI–TRUS fusion-guided biopsies using the Eigen/Artemis system with VE targeting [34]. They found that MRI–TRUS fusion-guided biopsies had a slightly improved CDR compared with VE for all cancers (32% vs. 26.7%, P = 0.1374) and for GS ≥ 3 + 4 (20.3% vs. 15.1%, P = 0.0523).
Puech et al. found no difference in the CDR of PCa for rigid software coregistration using MedCom Navigator versus cognitive fusion TB (53% vs. 47%). Furthermore, no variations in cancer positivity were observed in the categories of posterior (46 of 79, 58%), anterior (33 of 79, 42%), or smallest (25 of 79, 32%) MRI targets [15]. In 2013, Bjurlin et al. conducted a literature review, and their findings indicated that the use of MRI to target prostate biopsies can reduce sampling errors associated with conventional biopsy by providing better disease localization and sampling. They discovered that increased cancer sampling allows more accurate risk classification, which may influence therapeutic decision making. However, the best clinical application of MRB has not yet been determined [3].
EARLY DETECTION OF PROSTATE CANCER
The US Preventive Services Task Force (USPSTF) updated its recommendations in 2018, largely based on the publication of longer follow-up data from large screening trials and emerging evidence that the use of AS in low-risk PCa reduces the harms associated with screening overtreatment. The USPSTF now recommends that men aged 55–69 years undergo PSA testing following a discussion with their clinicians about the relative benefits and harms, although it still advises against PSA screening in younger men (aged 40–55 years) or those aged >70 years [35].
In the absence of widespread organized PSA testing, opportunistic testing has become a routine practice in many EU member countries. However, a recent study revealed reveals that while this strategy has minimal influence on PCa-specific mortality, it is associated with overdiagnosis than organized risk-adapted PSA testing [36]. This lack of effect is largely attributed to testing individuals who will not benefit (e.g., those with a 10-year life expectancy) without sufficient informed decision making [37], as well as repeated testing in men who are not at risk of developing severe PCa [38].
Overdiagnosis can be reduced by employing a risk-adapted detection strategy based on PSA values in conjunction with risk calculators and mpMRI, which can distinguish between significant and insignificant PCa and modify treatment accordingly. As a result, many cases of early PCa diagnosis can be handled with AS, thereby avoiding overtreatment [39]. Those with a lower-risk profile may benefit from local treatment, which has fewer adverse effects and produces better results than if the condition was diagnosed and treated later, thereby enhancing or maintaining the patient’s quality of life [40].
The EAU has created an algorithm to explain a risk-adapted strategy for PCa detection. This algorithm is intended for use in well-informed men aged >50 years with a life expectancy longer than 10–15 years. This algorithm clearly demonstrates how to achieve early detection of serious PCa while avoiding overdiagnosis and overtreatment. Following a clinical risk assessment and appropriate counseling, the PSA test represents the first step in identifying a large proportion of men with a low PSA value who do not require any further immediate investigations for 2–4 years (for those with a PSA value of 1–3 ng/mL) or 5 years (for those with a PSA value of 1 ng/mL and aged 60 years) [39].
A risk stratification nomogram (which considers factors such as age, family history, digital rectal examination, and prostate volume [PSA density] in a risk calculator) will identify a subgroup of men (approximately 35% of all men with an initial PSA test of >3 ng/mL [41]) as those with a low risk who require clinical follow-up only, avoiding the need for further testing, including MRI and biopsy. Men with a PSA value of >3 ng/mL who were classified as intermediate or high risk would then undergo mpMRI, leading to the identification of a further subgroup (approximately 54% of all men undergoing MRI [41]) with a Prostate Imaging Reporting and Data System (PIRADS) score of 1–2 considered at a low risk of having sPCa and requiring clinical follow-up only.
Further risk categorization of men with a PIRADS score of 3 using PSA density and other clinical characteristics would reveal an additional category requiring only clinical follow-up (a PIRADS score of 1, 2 or “low-risk” 3 accounts for approximately 57% of men tested by PIRADS [41]). As a result, the remaining subset of the original population could be regarded as intermediate or high risk and should undergo targeted and/or systematic biopsy. Within this subgroup, those with a positive diagnosis and a favorable grading group (approximately 25% of all confirmed diagnoses [41]) may be eligible for AS rather than active treatment.
However, as part of a collaborative decision-making process, all final treatment decisions should consider the patient’s values and preferences [40]. This algorithm demonstrates how PSA testing can be used more intelligently by incorporating risk calculators, such as those developed by the European Randomised Study of Screening for PC (ERSPC) and the Prostate Cancer Prevention Trial [42], as well as mpMRI and the Pirads score [43, 44], to reduce the number of men undergoing biopsy. The proposed time intervals for repeat PSA testing depending on age and initial PSA test result reflect the risk of a future clinically significant cancer diagnosis [45] and thus help reduce false-positive biopsies.
The risk calculator to be used must also be carefully selected. Although the ERSPC risk calculator has been well verified and may thus be regarded as superior, a recalibration step may be required to account for regional variations in prevalence and the link between PSA and PCa risk [46].
CANCER RISK ASSESSMENT USING TARGET BIOPSY
The change from systematic biopsy to image TB raises significant concerns regarding the clinical care of PCa. More men should choose AS if new biopsy techniques provide them with greater confidence.
However, the use of targeted biopsies will be exploited to justify additional overtreatment. Currently, risk classification systems based on biopsy results greatly affect therapy decisions [47]. These systems evolved from traditional systematic biopsies. When tumors are sampled more thoroughly with TB, the proportion of positive cores and maximum CCL are higher than with traditional biopsy [48, 18].
As a result, compared with systematic biopsy, TB increased risk attribution. In a computer simulation study that included 107 reconstructed 3D models of whole-mount prostatectomy specimens, Robertson et al. [49] revealed this critical issue. They discovered that a 12-core TRUS biopsy properly categorized only 24% of clinically significant cancer-containing prostates as high risk, compared with 74% of cases using a transperineal TB with four cores. Furthermore, the targeted biopsies had a higher proportion of positive cores and higher maximum CCLs. They concluded that when risk models derived from conventional TRUS biopsy are used for image-directed biopsy, there is a systematic rise in risk attribution.
At UCLA, one hundred and ninety-four AS men were treated with MR-US fusion TB, which included systematic and targeted sampling. Using only systematic biopsy and the Epstein histological criteria (Gleason score 6, 2 cores cancer, and 50% of any core), 28% of men were categorized as poor candidates for surveillance on confirmatory biopsy.
Incorporating TB increased the number of reclassified patients to 41%. In some circumstances, this is caused by the discovery of additional dangerous malignancies, whereas in others (i.e., numerous malignant cores from a single MRI target), it is the result of using a classification system that does not consider TB.
Given the inflation in risk attribution, TB may be used to justify aggressive treatment of more men, exacerbating the overtreatment problem. To avoid this unwanted effect, new risk stratification criteria based on image-targeted biopsy must be developed and verified. Consider a man who underwent a conventional biopsy with a low-volume GS of 3 + 3 and a TB with a low-volume GS of 3 + 4. TB combined with device-based tracking of malignant detects could be used to safely follow tumors that are now thought to require treatment [47].
INCORPORATION OF MULTIPARAMETRIC MAGNETIC RESONANCE IMAGING FUSION-GUIDED BIOPSY WITH RISK MODELING FOR PROSTATE CANCER
When compared with RP specimens, mpMRI detects 85%–95% of index lesions and sPCa [50]. The use of TB of dubious mpMRI lesions in a fusion biopsy context increases the identification of sPCa by 30% [22].
Multivariate risk-based techniques have been established to identify men with sPCa while avoiding needless biopsies [51, 52]. A risk calculator based on data from the ERSPC was created to quantify the sPCa risk. Roobol et al. revealed that in men with a PCa risk of 12.5%, 33% of routine biopsies might be avoided [52].
Although TB of mpMRI-suspicious lesions alone is a potential technique for reducing the overdetection of minor illnesses, MRI-invisible sPCa can be missed [22, 53, 54].
Unlike Alberts et al., Radtke et al. and van Leeuwen et al. developed risk calculators and added prebiopsy mpMRI to clinical parameters to determine an individual sPCa risk using a validated biopsy approach combining fusion-guided TB and transperineal systematic SBs as reference on the one hand and transperineal mapping and TB plus 12-core TRUS on the other [55, 56].
In the area under the curve (AUC) of the receiver operating characteristics curve analysis, Van Leeuwen et al. demonstrated that a model combining age, PSA, DRE, prostate volume, previous biopsy result, and mpMRI Pirads Likert score outperformed the model of clinical parameters alone with discrimination of 0.90 [56]. In addition to the model for biopsy-naive men, Radtke et al. internally validated a risk model for men with a previous negative biopsy that combined PSA, prostate volume, DRE, age, and mpMRI Pirads Likert scoring [55]. When compared with a validated clinical parameter risk calculator and Pirads, the model beat both instruments [55]. When risk models that include mpMRI and clinical parameters are compared with risk models that only use clinical parameters or PIRADS, the accuracy of the decision to perform a biopsy in a patient with sPCa suspicion can be increased. In conclusion, risk models that include mpMRI are superior to risk models that exclude mpMRI not only for men before initial biopsy but also for patients who have had previous negative biopsy results [55, 56].
While detection of sPCa can be improved, an unsuspicious mpMRI or a low PIRADS score cannot be used to justify not proceeding with a biopsy in the case of PCa suspicion. MRI fusion biopsy was claimed to help diagnose indolent PCa. However, finding low-risk PCa can improve patient safety by avoiding unnecessary treatment and improving disease monitoring accuracy and reliability when selecting individuals for AS.
Avoiding Multuparametric Magnetic Resonance Imaging Fusion Biopsy Failure
Although mpMRI offers helpful details regarding the diagnostic route for sPCa, mpMRI fusion biopsy can fail. To date, four potential mechanisms for mpMRI fusion biopsy failure have been identified: mpMRI-invisible cancer, improper sampling, mpMRI reader oversight, and intralesion GS heterogeneity [57]. Muthigi et al. demonstrated that in 71% of cases when SB discovered sPCa but TB did not, the malignant finding was within the sextant of the target lesion, correlating with the findings of Cash et al., who identified inaccurate sampling as one of the primary causes of fusion biopsy failure [58].
Similarly, Bryk et al. identified a combination of TB and ipsilateral SB as the best strategy to detect sPCa and avoid detection of low-risk PCa in patients with unilateral mpMRI lesions using TB, and both sided SB as a reference [59]. The findings of these two studies imply that increasing the number of samples taken from the target area can help minimize erroneous sampling and intralesion GS heterogeneity. However, Porpiglia et al. discovered that two targeted cores inserted in the center of the lesion are sufficient to precisely portray the index lesion [60].
More research on this topic is required. The mpMRI fusion biopsy failure caused by mpMRI-invisible cancer, which has a consistent negative predictive value (NPV) for mpMRI of 63%–98%, can only be solved with further SB [61, 62]. However, most groups that combined TB with 12-core SB found no substantial benefit in detecting sPCa by combining both approaches over TB alone [22, 63].
In contrast, Filson et al. discovered that the combination biopsy approach detected much more sPCa than TB or SB alone [64].
These controversial results lead to the conclusion that the superiority of sPCa detection in a combined biopsy approach compared to a TB-only approach increases with the amount of SB, but with the risk of finding significantly lower-risk diseases.
The question of whether to omit SB might never be entirely solved, and decisions should be made individually according to biopsy indications and patient needs.
The technique used to perform the biopsy is another point regarding the quality and possible reasons for failure of mpMRI fusion biopsy.
MULTIPARAMETRIC MAGNETIC RESONANCE IMAGING FUSION-GUIDED BIOPSY IN MEN REQUIRING A REPEAT BIOPSY
Men with a previous negative biopsy and an ongoing suspicion of PCa should be continuously monitored. Prior sampling reduces overall disease incidence relative to a biopsy-naive group; however, individuals with continuous suspicion for PCa suffer from inadequate NPV of 12-core TRUS-guided biopsy. MpMRI has been proven in many studies to be effective in monitoring this patient group and should thus be suggested in a repeat biopsy setting [65, 1].
Most recent studies have examined these patients as a subset of a larger cohort; however, some studies have paid special attention to this patient group: Simmons et al. evaluated the diagnostic accuracy of mpMRI in men requiring repeat prostate biopsy (PICTURE study), although only 31% of men had a previous negative biopsy [66]. When used as a positive test result, an mpMRI score of 3 has a sensitivity of 97%, specificity of 22%, NPV of 91%, and positive predictive value of 47% [66].
The authors conclude that a repeat biopsy can be avoided in 14% of men at the cost of missing 9% of sPCa [66]. Hansen et al. reported a significantly improved AUC when Pirads and PSA density were combined (0.82 vs. 0.85), implying that repeat biopsy should be avoided only in cases of worrying mpMRI and low PSA density [67]. Again, data are unclear on when it is safe to exclude SB. Arsov et al. compared in-bore TB to fusion-guided TB plus 12-core TRUS-SB in a prospective randomized experiment. They discovered that adding SB had no further benefit in detecting sPCa [10].
In contrast, recent papers comparing TB alone methods with 24- or 12-core SB reveal that TB alone misses a significant amount of sPCa [67].
MULTIPARAMETRIC MAGNETIC RESONANCE IMAGING FUSION-GUIDED BIOPSY IN MEN UNDER AS
Men with PCa who are eligible for AS are another important patient category because proper risk assessment of potentially less serious conditions is essential. To achieve this purpose, mpMRI in conjunction with fusion biopsy can aid in initial candidate screening and disease progression monitoring. Radtke et al. analyzed in a sample of 149 men whose initial mpMRI and fusion biopsy before AS resulted in significantly reduced rates of later AS qualifying (20% vs. 48%) during a 2-year follow-up compared with those selected for AS based on 12-core TRUS-guided biopsy [68].
These findings are supported by Henderson et al., who revealed in a prospective study that the apparent diffusion coefficient (ADC) is a good marker for choosing patients for AS because a low ADC value is associated with a shorter time to unfavorable histology [69]. Several recent studies have examined mpMRI and fusion biopsy for disease progression detection. Most of them reveal that mpMRI accurately predicts the likelihood of clinical progression and that patients with stable mpMRI findings have a low probability of developing the disease [70–72].
The inclusion of clinical criteria in the decision-making process appears to be advantageous when selecting patients for AS. Alberts et al. discovered in a cohort of 210 men without upgrade at baseline, confirmatory, or surveillance biopsy in instances of unsuspicious mpMRI and PSA density <0.15 ng/ mL, implying that follow-up biopsy should be avoided in these individuals [10]. However, whether following up with fusion biopsy confined to mpMRI-visible targets is sufficient is still being debated. Meng et al. and Frey et al. showed that on combined SB and TB follow-up mpMRI fusion biopsy, TB detected significantly more upgrades than SB, supporting the rationale for removing SB [70, 53].
Conversely, Tran et al., Ma et al., and Recabal et al. found that a significant fraction of higher-grade malignancies could only be diagnosed by SB, suggesting the necessity for additional SB [71, 72]. These contradictory results can be explained in part by differences in study parameters such as median TB and SB cores; however, they also highlight the need for additional research on long-term results, serial mpMRI for replacing repeat biopsies, and sufficiency of follow-up biopsies limited to mpMRI targets.
CONCLUSIONS
MRTB improves the quality of histological results compared with other approaches, with a correct detection of approximately 90% of significant index lesions.
The systematic 12-core TRUS-guided biopsy is still a widely used technique despite its limited sensitivity for the clinical detection of sPCa.
MRI-guided biopsies provide a higher detection rate for clinically detected sPCa and increase the percentage of positive cores.
Although currently used in patients who remain at high clinical suspicion of PCa despite a negative TRUS-guided systematic biopsy, with the increasing use of upfront diagnostic MRI, these biopsies are expected to replace standard systematic biopsies.
Correct staging allows the selection of the best treatment options, including focal treatments, and adequate evaluation of the prognosis. It also reduces the incidence of re-biopsy, costs, and complications.
It is crucial to standardize the bioptic technique and improve the fusion technique to reduce understaging caused by errors in estimating the tumor volume. The role of mpMRI in the presurgical phase of RP is emerging because mpMRI can help in planning the initial surgical strategy, referring to clinical decision making.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
Авторлар туралы
Valentina Testini
Foggia University; Monsignor Raffaele Dimiccoli
Email: testinivalentina@gmail.com
ORCID iD: 0000-0003-1231-5213
MD
Италия, Foggia; BarlettaLaura Eusebi
Carlo Urbani Hospital
Email: lauraeu@virgilio.it
ORCID iD: 0000-0002-4172-5126
MD
Италия, JesiFrancesco Guerra
Foggia University
Email: francesco.rino@gmail.com
ORCID iD: 0000-0003-3923-3429
MD
Италия, FoggiaWilly Giannubilo
Ospedale Civile
Email: willygiannubilo@virgilio.it
MD
Италия, Civitanova MarcheManuel Di Biase
Santa Maria della Misericordia Hospital
Email: manuel.dibiase@ospedale.perugia.it
MD
Италия, PerugiaAnnunziata Russo
Monsignor Raffaele Dimiccoli
Email: tittyrusso-23@libero.it
MD
Италия, BarlettaGiuseppe Guglielmi
Foggia University; Monsignor Raffaele Dimiccoli; Casa Sollievo della Sofferenza Hospital
Хат алмасуға жауапты Автор.
Email: giuseppe.guglielmi@unifg.it
ORCID iD: 0000-0002-4325-8330
MD, Professor
Италия, Foggia; Barletta; FoggiaӘдебиет тізімі
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003
- Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–719. doi: 10.1016/j.eururo.2013.05.059
- Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014;192(3):648–658. doi: 10.1016/j.juro.2014.03.117
- [WITHDRAWN] Prostate cancer risk management programme (PCRMP): benefits and risks of PSA testing (guidance) [Internet]. UK: Public Health England; 2016. Available from: https://www.gov.uk/government/publications/prostate-cancer-risk-management-programme-psa-test-benefits-and-risks
- Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol. 2015;67(6):1112–1121. doi: 10.1016/j.eururo.2014.10.033
- Ventrella E, Eusebi L, Carpagnano FA, et al. Multiparametric MRI of Prostate Cancer: Recent Advances. Curr Radiol Rep. 2020;8. doi: 10.1007/s40134-020-00363-1
- Portalez D, Mozer P, Cornud F, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol. 2012;62(6):986–996. doi: 10.1016/j.eururo.2012.06.044
- Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int. 2013;112(8):1080–1087. doi: 10.1111/bju.12259
- Carpagnano F, Eusebi L, Tupputi U, et al. Multiparametric MRI: Local Staging of Prostate Cancer. Current Radiology Reports. 2020;8. doi: 10.1007/s40134-020-00374-y
- Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68(4):713–720. doi: 10.1016/j.eururo.2015.06.008
- Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183(2):520–527. doi: 10.1016/j.juro.2009.10.022
- Tyson MD, Arora SS, Scarpato KR, Barocas D. Magnetic resonance-ultrasound fusion prostate biopsy in the diagnosis of prostate cancer. Urol Oncol. 2016;34(7):326–332. doi: 10.1016/j.urolonc.2016.03.005
- Wolters T, Montironi R, Mazzucchelli R, et al. Comparison of incidentally detected prostate cancer with screen-detected prostate cancer treated by prostatectomy. Prostate. 2012;72(1):108–115. doi: 10.1002/pros.21415
- Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63(1):125–140. doi: 10.1016/j.eururo.2012.06.004
- Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy — prospective multicenter study. Radiology. 2013;268(2):461–469. doi: 10.1148/radiol.13121501
- Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010;13(1):65–70. doi: 10.1038/pcan.2009.41
- Williams IS, McVey A, Perera S, et al. Modern paradigms for prostate cancer detection and management. Med J Aust. 2022;217(8):424–433. doi: 10.5694/mja2.51722
- Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108(8 Pt 2):E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x
- Labanaris AP, Zugor V, Smiszek R, et al. Guided e-MRI prostate biopsy can solve the discordance between Gleason score biopsy and radical prostatectomy pathology. Magn Reson Imaging. 2010;28(7):943–946. doi: 10.1016/j.mri.2010.03.041
- Stephenson SK, Chang EK, Marks LS. Screening and detection advances in magnetic resonance image-guided prostate biopsy. Urol Clin North Am. 2014;41(2):315–326. doi: 10.1016/j.ucl.2014.01.007
- Vourganti S, Rastinehad A, Yerram N, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188(6):2152–2157. doi: 10.1016/j.juro.2012.08.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397. doi: 10.1001/jama.2014.17942
- Quentin M, Schimmöller L, Arsov C, et al. 3-T in-bore MR-guided prostate biopsy based on a scoring system for target lesions characterization. Acta Radiol. 2013;54(10):1224–1229. doi: 10.1177/0284185113492972
- Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012;62(5):902–909. doi: 10.1016/j.eururo.2012.01.047
- Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23(1):43–50. doi: 10.1097/MOU.0b013e32835ad3ee
- Monni F, Fontanella P, Grasso A, et al. Magnetic resonance imaging in prostate cancer detection and management: a systematic review. Minerva Urol Nefrol. 2017;69(6):567–578. doi: 10.23736/S0393-2249.17.02819-3
- Schlaier JR, Warnat J, Dorenbeck U, et al. Image fusion of MR images and real-time ultrasonography: evaluation of fusion accuracy combining two commercial instruments, a neuronavigation system and a ultrasound system. Acta Neurochir (Wien). 2004;146(3):271–276. doi: 10.1007/s00701-003-0155-6
- Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66(1):22–29. doi: 10.1016/j.eururo.2014.03.002
- Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190(4):1380–1386. doi: 10.1016/j.juro.2013.04.043
- Serefoglu EC, Altinova S, Ugras NS, et al. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer. Can Urol Assoc J. 2013;7(5-6):E293–E298. doi: 10.5489/cuaj.11224
- Baco E, Ukimura O, Rud E, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67(4):787–794. doi: 10.1016/j.eururo.2014.08.077
- Cerantola Y, Dragomir A, Tanguay S, et al. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol. 2016;34(3):119.e1–119.e9. doi: 10.1016/j.urolonc.2015.09.010
- Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189(2):493–499. doi: 10.1016/j.juro.2012.08.195
- Bax J, Cool D, Gardi L, et al. Mechanically assisted 3D ultrasound guided prostate biopsy system. Med Phys. 2008;35(12):5397–5410. doi: 10.1118/1.3002415
- US Preventive Services Task Force; Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901–1913. Erratum in: JAMA. 2018;319(23):2443. doi: 10.1001/jama.2018.3710
- Arnsrud Godtman R, Holmberg E, Lilja H, Stranne J, Hugosson J. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Göteborg randomized population-based prostate cancer screening trial. Eur Urol. 2015;68(3):354–360. doi: 10.1016/j.eururo.2014.12.006
- Engler J, Dahlhaus A, Güthlin C. The readiness of German GPs to recommend and conduct cancer screening is associated with patient-physician gender concordance. Results of a survey. Eur J Gen Pract. 2017;23(1):11–19. doi: 10.1080/13814788.2016.1240166
- Nordström T, Aly M, Clements MS, et al. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003-2011. Eur Urol. 2013;63(3):419–425. doi: 10.1016/j.eururo.2012.10.001
- Van Poppel H, Roobol MJ, Chapple CR, et al. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur Urol. 2021;80(6):703–711. doi: 10.1016/j.eururo.2021.07.024
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042
- Collen S, Van Poppel H. Early detection and diagnosis of prostate cancer in well informed men: the way forward for Europe. Belg J Med Oncol. 2020;14:321–326.
- Louie KS, Seigneurin A, Cathcart P, Sasieni P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol. 2015;26(5):848–864. doi: 10.1093/annonc/mdu525
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822. doi: 10.1016/S0140-6736(16)32401-1
- Alberts AR, Schoots IG, Bokhorst LP, et al. Characteristics of Prostate Cancer Found at Fifth Screening in the European Randomized Study of Screening for Prostate Cancer Rotterdam: Can We Selectively Detect High-grade Prostate Cancer with Upfront Multivariable Risk Stratification and Magnetic Resonance Imaging. Eur Urol. 2018;73(3):343–350. doi: 10.1016/j.eururo.2017.06.019
- Palsdottir T, Nordstrom T, Karlsson A, et al. The impact of different prostate-specific antigen (PSA) testing intervals on Gleason score at diagnosis and the risk of experiencing false-positive biopsy recommendations: a population-based cohort study. BMJ Open. 2019;9(3):e027958. doi: 10.1136/bmjopen-2018-027958
- Wynants L, van Smeden M, McLernon DJ, et al. Three myths about risk thresholds for prediction models. BMC Med. 2019;17(1):192. doi: 10.1186/s12916-019-1425-3
- Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014;32(6):903–911. doi: 10.1016/j.urolonc.2013.08.006
- Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–91. doi: 10.1016/j.juro.2012.08.095
- Robertson NL, Hu Y, Ahmed HU, et al. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol. 2014;65(3):628–634. doi: 10.1016/j.eururo.2012.12.057
- Radtke JP, Schwab C, Wolf MB, et al. Multiparametric magnetic resonance imaging (MRI) and MRI — transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016;70:846–853. doi: 10.1016/j.eururo.2015.12.052
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0
- Roobol MJ, Steyerberg EW, Kranse R, et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol. 2010;57(1):79–85. doi: 10.1016/j.eururo.2009.08.025
- Meng X, Rosenkrantz AB, Mendhiratta N, et al. Relationship Between Prebiopsy Multiparametric Magnetic Resonance Imaging (MRI), Biopsy Indication, and MRI-ultrasound Fusion-targeted Prostate Biopsy Outcomes. Eur Urol. 2016;69(3):512–517. doi: 10.1016/j.eururo.2015.06.005
- Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2016;26(6):1606–1612. doi: 10.1007/s00330-015-4015-6
- Radtke JP, Wiesenfarth M, Kesch C, et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer-Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies. Eur Urol. 2017;72(6):888–896. doi: 10.1016/j.eururo.2017.03.039
- van Leeuwen PJ, Hayen A, Thompson JE, et al. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120(6):774–781. doi: 10.1111/bju.13814
- Muthigi A, George AK, Sidana A, et al. Missing the Mark: Prostate Cancer Upgrading by Systematic Biopsy over Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Biopsy. J Urol. 2017;197(2):327–334. doi: 10.1016/j.juro.2016.08.097
- Cash H, Günzel K, Maxeiner A, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int. 2016;118(1):35–43. doi: 10.1111/bju.13327
- Bryk DJ, Llukani E, Taneja SS, et al. The Role of Ipsilateral and Contralateral Transrectal Ultrasound-guided Systematic Prostate Biopsy in Men With Unilateral Magnetic Resonance Imaging Lesion Undergoing Magnetic Resonance Imaging-ultrasound Fusion-targeted Prostate Biopsy. Urology. 2017;102:178–182. doi: 10.1016/j.urology.2016.11.017
- Porpiglia F, De Luca S, Passera R, et al. Multiparametric Magnetic Resonance/Ultrasound Fusion Prostate Biopsy: Number and Spatial Distribution of Cores for Better Index Tumor Detection and Characterization. J Urol. 2017;198(1):58–64. doi: 10.1016/j.juro.2017.01.036
- Fütterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol. 2015;68(6):1045–1053. doi: 10.1016/j.eururo.2015.01.013
- Thompson JE, Moses D, Shnier R, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol. 2014;192(1):67–74. doi: 10.1016/j.juro.2014.01.014
- Delongchamps NB, Portalez D, Bruguière E, et al. Are Magnetic Resonance Imaging-Transrectal Ultrasound Guided Targeted Biopsies Noninferior to Transrectal Ultrasound Guided Systematic Biopsies for the Detection of Prostate Cancer. J Urol. 2016;196(4):1069–1075. doi: 10.1016/j.juro.2016.04.003
- Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122(6):884–892. doi: 10.1002/cncr.29874
- Rosenkrantz AB, Verma S, Choyke P, et al. Prostate Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Patients with a Prior Negative Biopsy: A Consensus Statement by AUA and SAR. J Urol. 2016;196(6):1613–1618. doi: 10.1016/j.juro.2016.06.079
- Simmons LAM, Kanthabalan A, Arya M, et al. The PICTURE study: diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br J Cancer. 2017;116(9):1159–1165. doi: 10.1038/bjc.2017.57
- Hansen NL, Kesch C, Barrett T, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int. 2017;120(5):631–638. doi: 10.1111/bju.13711
- Radtke JP, Kuru TH, Bonekamp D, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared with standard 12-core systematic biopsies for the selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis. 2016;19(3):283–291. doi: 10.1038/pcan.2016.16
- Henderson DR, de Souza NM, Thomas K, et al. Nine-year Follow-up for a Study of Diffusion-weighted Magnetic Resonance Imaging in a Prospective Prostate Cancer Active Surveillance Cohort. Eur Urol. 2016;69(6):1028–1033. doi: 10.1016/j.eururo.2015.10.010
- Frye TP, George AK, Kilchevsky A, et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol. 2017;197(3 Pt 1):640–646. doi: 10.1016/j.juro.2016.08.109
- Recabal P, Assel M, Sjoberg DD, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol. 2016;196(2):374–381. doi: 10.1016/j.juro.2016.02.084
- Tran GN, Leapman MS, Nguyen HG, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol. 2017;72(2):275–281. doi: 10.1016/j.eururo.2016.08.023
Қосымша файлдар