Structural gray matter changes in primary progressive aphasia variants
- Autores: Akhmadullina D.R.1, Konovalov R.N.1, Shpilyukova Y.A.1, Fedotova E.Y.1
-
Afiliações:
- Research Center of Neurology
- Edição: Volume 4, Nº 4 (2023)
- Páginas: 467-480
- Seção: Original Study Articles
- ##submission.dateSubmitted##: 27.07.2023
- ##submission.dateAccepted##: 22.08.2023
- ##submission.datePublished##: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/567783
- DOI: https://doi.org/10.17816/DD567783
- ID: 567783
Citar
Resumo
BACKGROUND: Primary progressive aphasia is a rare neurodegenerative disease with high clinical, genetic, and pathomorphological heterogeneity that greatly complicates its diagnosis. Voxel-based morphometry can be used to objectively assess structural gray matter changes and determine atrophy patterns in variants of primary progressive aphasia, which can improve the diagnosis and our understanding of its pathogenesis.
AIMS: This study aimed to evaluate the patterns of atrophy in each of the primary progressive aphasia variants in comparison with the control group.
MATERIALS AND METHODS: Patients diagnosed with one of the primary progressive aphasia variants, established in accordance with the current diagnostic criteria, were included in the main group. The control group consisted of healthy volunteers without any neurological symptoms or structural brain changes. All participants underwent brain magnetic resonance imaging. The obtained images were processed and used for voxel-based morphometry, which was performed by comparing the gray matter volume between each of the primary progressive aphasia variants and the control group. The study was adjusted for the sex, age, and intracranial volume of the participants.
RESULTS: The study enrolled 25 patients with nonfluent, 11 with semantic, and 9 with logopenic variants of primary progressive aphasia, as well as 20 healthy volunteers. Voxel-based morphometry showed a specific atrophy pattern in each of the variants of primary progressive aphasia, with predominant involvement of the frontal and insular lobes in nonfluent, temporal lobe and hippocampus in semantic, and a more diffuse frontotemporal pattern in logopenic variants.
CONCLUSIONS: The study revealed gray matter atrophy patterns specific to each variant of primary progressive aphasia. The obtained results mainly correspond to the clinical presentations of the disease. Moreover, some findings (e.g., absence of the posterior perisylvian atrophy and reduced motor cortex volume in the logopenic variant, atrophy of the orbitofrontal cortex and cerebellum in the nonfluent variant, and premotor cortex, precentral, and inferior frontal gyrus degeneration in the semantic variant) do not correlate with the usual understanding of primary progressive aphasia pathogenesis and require further study.
Texto integral
ABBREVIATIONS
AV-PPA: agrammatic variant of primary progressive aphasia
FTD: frontotemporal dementia
GM: gray matter
IFG: inferior frontal gyrus
ITG: inferior temporal gyrus
LV-PPA: logopenic variant of primary progressive aphasia
MNI: Montreal Neurological Institute
mPFC: medial prefrontal cortex
MRI: magnetic resonance imaging
MTG: middle temporal gyrus
OFC: orbitofrontal cortex
PPA: primary progressive aphasia
SMA: supplementary motor area
STG: superior temporal gyrus
SV-PPA: semantic variant of primary progressive aphasia
VBM: voxel-based morphometry
BACKGROUND
Primary progressive aphasia (PPA) is a neurodegenerative disease characterized by early, constantly progressive speech disorders in the absence of other cognitive, motor, and/or behavioral disorders. PPA refers to early-onset dementias (<65 years) and, despite its low incidence, presents a relevant socioeconomic problem. Three clinical variants of PPA are distinguished based on clinical presentation: agrammatic (AV-PPA), semantic (SV-PPA), and logopenic (LV-PPA) variants. AV- and SV-PPA are usually a sign of frontotemporal dementia (FTD), whereas LV-PPA indicates atypical Alzheimer’s disease. However, this distinction is not definitive because any PPA variant may demonstrate different pathomorphological and genetic variants, which results in diverse clinical presentations of the disease and complicates its diagnosis.
Apart from a neurological examination, neuroimaging is the only approved method for the differential diagnosis of PPA variants. A previous study helped identify specific involvement areas for each PPA variant, which was included in the 2011 diagnostic criteria [1]:
- AV-PPA is mainly characterized by atrophy of posterior frontal areas—inferior frontal gyrus (IFG), premotor cortex, and supplementary motor area (SMA) — and of the insula, prevailing on the left side.
- In SV-PPA, atrophy of the anterior–inferior sections of the left temporal lobe is typical.
- In LV-PPA, posterior perisylvian areas and/or the parietal lobe of the left hemisphere are commonly involved.
A later meta-analysis verified the presence of a specific pattern of neural degeneration in each PPA variant; however, the involvement appeared to be more extensive, including medial areas of the temporal lobes in SV-PPA; precentral gyrus, superior gyrus (STG), and middle temporal gyrus (MTG) in AV-PPA; and posterior cingulate cortex in LV-PPA [2]. However, the number of studies on gray matter (GM) involvement in PPA remains limited. To illustrate, the meta-analysis mentioned above included only 20 papers, with the data of 317 patients (of which 169, 90, and 58 had SV-PPA, AV-PPA, and LV-PPA, respectively). In addition, many studies included were conducted using outdated diagnostic criteria, which makes the relevance of the results questionable, particularly for AV-PPA and LV-PPA. In recent years, larger studies have suggested that GM involvement in PPA is probably more extensive than previously thought; however, the atrophy patterns identified often differ [3, 4]. In addition, the clinical signs of PPA variants may vary in different populations because of language differences, which in turn may result in differences in the underlying GM degeneration [5]. The only paper evaluating structural changes of the brain in PPA in the Russian population included patients with AV-PPA exclusively, and no studies have focused on SV-PPA and LV-PPA when this paper was being written [6].
Meanwhile, neuroimaging methods are increasingly used for the diagnosis, evaluation, and follow-up of patients with PPA. For instance, machine learning based on the data of structural magnetic resonance imaging (MRI) may be used for the differential diagnosis of PPA variants and FTD and for the more extensive differential diagnosis of neurodegenerative dementias. Moreover, neuroimaging may be used to evaluate the therapeutic effect of novel treatment modalities [3, 7, 8]. All of the above emphasizes the relevance of such studies.
AIM
This study aimed to characterize the atrophy patterns in each of the PPA variants in the Russian population and compare the data obtained with those of previous studies.
METHODS
Study design
This was an experimental, single-center, cross-sectional study.
Eligibility
Subjects were enrolled in the study on the basis of their compliance with the inclusion/non-inclusion criteria.
Inclusion criteria for the experimental group (PPA group): age >18 years and diagnosis of one of the PPA variants based on the current diagnostic criteria [1].
Inclusion criteria for the control group: age >18 years and absence of neurological symptoms.
Noninclusion criteria: MRI contraindications and structural focal changes in the brain.
Site
The study was conducted at the Research Center of Neurology (Moscow).
Study duration
The subjects were recruited from 2022 to 2023.
Medical intervention
Addenbrooke’s Cognitive Examination Revised was used to evaluate cognitive functions in the PPA group. Emotional and behavioral disorders were evaluated using a neuropsychiatric questionnaire. Disease severity was assessed using the FTD severity scale.
Brain MRI in the 3D-T1 MPR sequence using Magnetom Verio or Magnetom Prisma at a field magnitude of 3 Tesla was performed for all study subjects.
The MR images obtained were used for voxel-based morphometry (VBM).
SPM12 software (Institute of Neurology, UK) based on Matlab R2019b (MathWorks Inc., Natick, MA, USA) was used for postprocessing and statistical analysis. Postprocessing involved the following:
- Normalizing the images to the same MNI stereotaxic space (3D system of coordinates of the human brain by the Montreal Neurological Institute)
- Segmentation into GM, white matter, and cerebrospinal fluid using the DARTEL algorithm
- Further smoothing of the images was obtained with an isotropic Gaussian kernel with full width at a half height of 8 mm.
VBM results were assessed for every PPA variant versus the control group. The two-sample t-test with voxel-wise comparison was used for the study groups. Exclusive analysis of GM was possible using a GM mask generated specifically for each group. The age and sex of the participants were used as covariates. The study was performed with adjustment for intracranial volume, which was measured as the sum of GM, white matter, and cerebrospinal fluid volumes. Clusters with a minimum volume of 100 voxels were included in the analysis. The cutoff for the inclusion of individual voxels into clusters was set at a level of P < 0.05 with an adjustment for the expected percentage of false rejections.
The bspmview software was used for VBM result visualization, presentation of the statistical data, and coordinate localization [9].
Ethical evaluation
This study was approved by the Ethics Committee of the Research Center of Neurology (Protocol No. 11-7/22 dated December 21, 2022).
Statistical analysis
IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Differences in nominal and ordinal variables were analyzed using Fisher’s exact test, and differences in quantitative variables were analyzed using the Kruskal–Wallis test with Bonferroni correction.
RESULTS
Study subjects
The study included 45 patients with PPA variants and 30 control subjects. Of the 45 patients, 25, 11, and 9 had AV-PPA, SV-PPA, and LV-PPA, respectively. The key characteristics of the study groups are summarized in Table 1. The median ages were 64, 67, and 65 years for the AV-PPA, SV-PPA, and LV-PPA groups, respectively. Female patients prevailed in the AV-PPA and SV-PPA groups, whereas male patients prevailed in the LV-PPA group. The disease duration ranged from 6 to 108 months, with the longest duration in the AV-PPA group. The disease severity ranged from very mild to severe, and mild to moderate symptoms were the most common. Despite the shorter disease duration, the most severe cognitive, emotional, and behavioral disorders were observed in SV-PPA.
Table 1. Clinical characteristics of the study groups
Index | AV-PPA (n=25) | SV-PPA (n=11) | LV-PPA (n=9) | Control group (n=30) |
Sex, M/F (%) | 9/16 (36%; 64%) | 5/6 (45%; 55%) | 6/3 (67%; 33%) | 10/20 (33%; 67%) |
Age, years | 64 [57; 67]* | 65 [56; 67] | 56 [51; 59]*,† | |
Disease duration, months | 48 [36; 60] | 36 [16; 48] | 36 [23; 48] | – |
АCE-R, total score/100 | 68 [36; 80] | 38 [26; 50] | 53 [37; 75] | – |
Neuropsychiatric questionnaire, score/144 | 8 [1; 14]* | – |
Notes: The data are described as Me [Q1; Q3]; ACE-R, Addenbrooke’s Cognitive Examination Revised; M, male; F, female; *,†: the difference between groups is statistically significant (P < 0.05).
Despite the described differences, no statistically significant differences in sex, age, disease duration, and severity of cognitive disorders were observed among the PPA variants.
No difference in the distribution by sex was observed against the control group; however, the control group was statistically significantly younger than the AV-PPA and SV-PPA groups.
Key findings
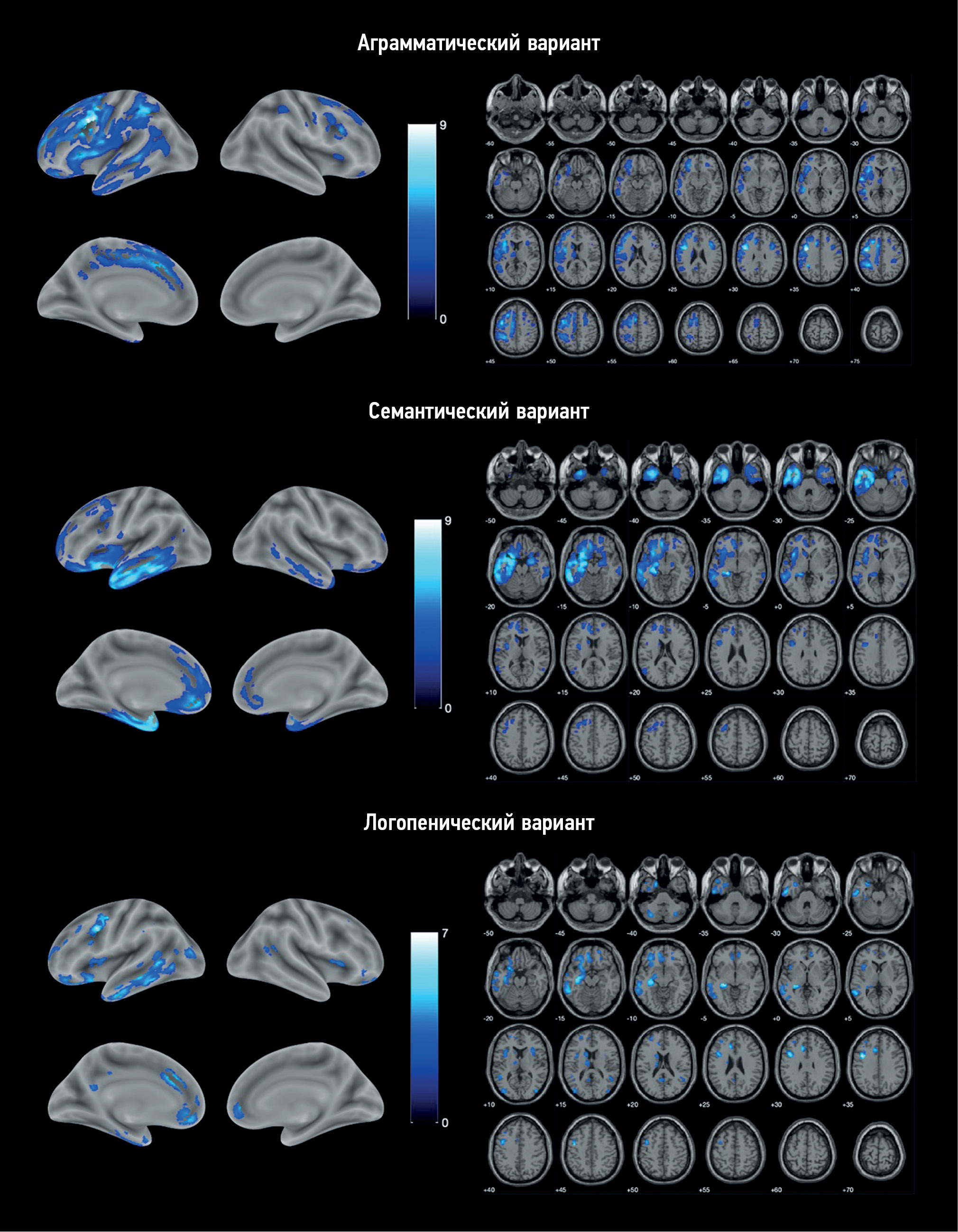
VBM identified areas of atrophy in each PPA variant compared with the control group (Fig. 1). Atrophy in all cases was asymmetric, prevailing in the left hemisphere.
Fig. 1. Localization of the areas of the loss of the gray matter volume in variants of primary progressive aphasia vs. control group. The color coding is for the T-value.
AV-PPA group: The atrophic “core” was localized in the left IFG and precentral gyrus (Table 2). Significant changes were also observed in the SMA, premotor cortex, orbitofrontal cortex (OFC), and insular cortex of both hemispheres. The temporal lobe was mainly involved in the MTG and inferior temporal gyrus (ITG), continuing into the area of the temporoparietal junction and inferior parietal lobule. In addition, atrophic involvement of subcortical structures was observed, namely, the left caudate nucleus, thalamus, putamen, and cerebellum.
Table 2. Areas of the loss of the gray matter volume in the group with agrammatic variant of primary progressive aphasia vs. control group
Brain area | Volume, voxels | MNI peak coordinates x, y, z |
Precentral gyrus, inferior frontal gyrus, supplementary motor area, insula, superior and middle frontal gyri, orbitofrontal cortex, cingulate cortex, lower parietal lobule, angular and supramarginal gyri, lateral areas of the temporal lobe, putamen, caudate nucleus, S | 37,644 | -40, 4, 34 |
-42, -2, 42 | ||
-57, -4, 4 | ||
Opercular and triangular parts of the IFG, D | 2,264 | 39, 18, 26 |
38, 6, 27 | ||
36, 4, 40 | ||
Caudate nucleus; thalamus, D | 1,065 | -16, -16, 22 |
-14, -12, 10 | ||
-10, 10, 16 | ||
OFC, D | 180 | 24, 38, -9 |
Cerebellar crus I, D | 101 | 18, -72, -36 |
Precuneus, S | 105 | -10, -57, 27 |
Postcentral gyrus, D | 314 | 33, -34, 38 |
36, -26, 39 | ||
Insula, D | 191 | 34, 20, 10 |
Precentral gyrus, D | 257 | 52, -6, 45 |
57, -6, 33 |
Note: D, on the right; S, on the left.
SV-PPA group: Atrophy was predominantly localized in the left temporal lobe, including its pole, inferior–lateral and medial regions, and left hippocampus and insula (Table 3). Individual lesions were observed in the left frontal lobe, including the OFC, medial prefrontal cortex (mPFC), premotor cortex, precentral gyrus, and IFG. Overall, the changes were more localized than in AV-PPA and were limited to the frontal, temporal, and insular cortices, except for an atrophic lesion in the left caudate nucleus. Similar but less extensive atrophic areas were identified in the right hemisphere.
Table 3. Areas of the loss of the gray matter volume in the group with semantic variant of primary progressive aphasia versus control group
Brain area | Volume, voxels | MNI peak coordinates x, y, z |
Hippocampus; lateral and medial areas of the temporal lobe; temporal pole; insula; anterior cingulate cortex; OFC; caudate nucleus, S; OFC, D | 36,682 | -24, -30, -4 |
-52, -46, -15 | ||
-56, -39, -16 | ||
Hippocampus, temporal lobe pole, ITG, OFC, D | 6,563 | 24, -6, -21 |
39, 10, -33 | ||
24, 9, -21 | ||
Middle regions of STG, S | 344 | -57, -6, 4 |
Premotor cortex, middle parts of the precentral gyrus, opercular part of IFG, S | 1,809 | -28, 10, 54 |
-40, 3, 32 | ||
-24, 6, 38 | ||
Posterior regions of MTG, S | 253 | -51, -68, 16 |
-44, -57, 15 | ||
mPFC, S | 125 | -9, 22, 48 |
Posterior regions of the MTG and ITG, D | 104 | 56, -62, 9 |
58, -54, -3 |
Note: D, on the right; S, on the left.
LV-PPA group: The most pronounced loss of the GM volume was also localized in the left temporal lobe but was mostly involved in the posterior parts of MTG and ITG and, to a lesser extent, the temporal pole. In addition, it continued into the parahippocampal gyrus, hippocampus, and amygdala (Table 4). Atrophy was the most pronounced in the precentral gyrus, anterior cingulate cortex, OFC, and mPFC. Apart from the frontal and temporal lobes, atrophy in this PPA variant involved the insular lobes, left parietal and occipital lobes, cerebellum, and left caudate nucleus.
Table 4. Areas of the loss of the gray matter volume in the group with logopenic variant of primary progressive aphasia versus control group
Brain area | Volume, voxels | MNI peak coordinates x, y, z |
Precentral gyrus, S | 1,304 | -40, 6, 34 |
-42, 0, 45 | ||
-36, 3, 52 | ||
Hippocampus, amygdala, ITG, MTG, OFC, S | 8,136 | -26, -30, -3 |
-36, -16, -15 | ||
-27, -24, -9 | ||
Anterior cingulate cortex, S | 501 | -12, 26, 27 |
Anterior cingulate cortex, S; mPFC, S and D | 1,130 | -10, 44, -14 |
14, 45, -2 | ||
-9, 38, -6 | ||
Cerebellar crus II, S | 325 | -32, -70, -39 |
-38, -60, -42 | ||
Middle occipital gyrus, S | 328 | -40, 82, 14 |
Caudate nucleus, S | 325 | -14, -10, 20 |
Insula and IFG, S | 537 | -14, 16, 8 |
-38, 4, 15 | ||
Temporal lobe pole, S | 218 | -45, -15, -36 |
Posterior regions of MTG, D | 166 | 46, -48, 15 |
Rostrolateral prefrontal cortex, S | 389 | -30, 51, 21 |
-21, 56, 10 | ||
-33, 42, 24 | ||
OFC, D | 110 | 20, 52, -14 |
Cerebellar crus I, D | 145 | 32, -66, -39 |
Caudate nucleus, S | 201 | -14, 6, 18 |
Precuneus, S | 170 | -8, -54, 18 |
Insula, D | 211 | 33, 18, 12 |
34, 9, 14 |
Note: D, on the right; S, on the left.
DISCUSSION
Key findings summary
The study revealed GM areas with the involvement typical of each PPA variant. The identified atrophy patterns were largely consistent with literature data, although certain specifics were discovered.
Key findings discussion
In AV-PPA, the GM was expectedly seen in the IFG, precentral gyrus, premotor cortex, SMA, and anterior insula, i.e., the areas in which atrophy has been repeatedly described in AV-PPA and correlates closely with speech disorders specific for this PPA variant [10]. For instance, the loss of the GM volume in the IFG correlated with the general severity of aphasia and agrammatisms; in the left insula, with the severity of speech fluency disorders; and atrophy of SMA and premotor cortex was associated with speech apraxia, articulation rate, and nonverbal oral apraxia [11–14]. Moreover, the degeneration of the precentral gyrus may be associated with concomitant AV-PPA through motor neuronal disease, which was found in 16% of patients with AV-PPA in our sample.
Apart from the frontal lobes, atrophy also spreads to the lateral regions of the left temporal lobe. Although its involvement is considered less “classic” in AV-PPA, it is present in most studies of structural changes in this variant. This might be indicative of progressive neurodegeneration over time and account for difficulties in understanding individual words and naming [2, 15, 16].
Bilateral OFC atrophy is of particular interest because it is extremely rare in AV-PPA and is more often associated with emotional and behavioral disorders. Mild-to-moderate behavioral disorders were observed in most patients in our sample, which could account for this finding. In addition, OFC atrophy was previously described in patients with PPA associated with GRN mutation, albeit only in nonclassifiable PPA cases, the clinical presentation of which does not match any of the variants [17]. GRN mutations were verified in two patients with AV-PPV from our sample; however, their clinical presentation was standard for this variant; therefore, the identified atrophy was not attributable to the genetic features of the group. This finding requires further studies in a larger sample in our region.
Atrophy in AV-PPV was observed in subcortical structures, such as the left thalamus, putamen, and caudate nucleus. Recently, more studies have reported thalamic atrophy in FTD variants, particularly in genetic disease forms; however, such changes are more typical of the behavioral variant of FTD than of AV-PPA, in which thalamic atrophy is more localized and is not observed in all cases [2, 16–19]. Atrophy of the putamen and caudate nucleus was previously described in single papers but not in larger studies [4, 10, 13]. Overall, despite the evidence of the role of the thalamus and basal ganglia in speech articulation and phonology because of their connections with frontal and parietal regions, there is no conclusive opinion on how their degeneration affects speech disorders in PPA [20].
Another outstanding finding in AV-PPA is that the cerebellum is reduced in size. Cerebellar atrophy in FTD was first described in the disease secondary to a C9ORF72 mutation; however, a more typical finding of this case was bilateral, relatively symmetric atrophy, which, apart from the cerebellum, also usually spreads to parietal and occipital areas, and we did not observe this in our study. Another possible explanation is the role of the cerebellum in speech functions. Previously, lobule VII of the cerebellar hemispheres (atrophy of which we identified in our study) is involved in feedback control in oral speech, and its significance is greater in the gradual disorganization of the speech regions of the brain [20].
The atrophy pattern identified in the SV-PPA group is largely consistent with literature data. The most significant reduction in the GM volume was observed in the temporal poles of both hemispheres, predominantly on the left side. The left temporal pole is a semantic hub from which verbal semantic information is stored, processed, and extracted. Its atrophy is the key sign of SV-PPA, and anomia and difficulty understanding individual words in this variant are associated with it [21]. Asymmetric atrophy of the hippocampus and medial and inferior regions of the temporal lobes identified in our study is another major sign of SV-PPA, which has been reported repeatedly. Notably, unlike Alzheimer’s disease, SV-PPA is characterized by atrophy of the anterior regions of the hippocampi, which also correlates with semantic deficit severity in patients [22]. The involvement of the inferior regions of the temporal lobes in SV-PPA, particularly the fusiform gyrus, correlates with emotional and behavioral disorders and prosopagnosia, whereas the involvement of the lateral regions of STG and MTG is associated with the severity of anomia, difficulty understanding individual words, and dyslexia severity [21, 23]. The loss in the volume of the anterior cingulate cortex, mPFC, OFC, insula, and caudate nucleus is typical of more advanced stages of SV-PPA and is associated with a gradual spread of the process from the left temporal pole to closely related areas [24, 25]. The involvement of these regions is associated with social activity disorders; however, only a few papers have focused on this subject [26]. Atrophy of the left IFGs, premotor cortex, and precentral gyrus, which we identified, is less common in SV-PPA. Such changes may cause gradual development of the clinical presentation consistent with the disease and occurrence of motor speech disorders [16].
The GM volume loss observed in LV-PPA was more diffuse, with multiple small degenerative lesions. The most prominent areas of atrophy in our sample were located in the left temporal and frontal lobes. The loss of MTG and ITG volume is often reported in LV-PPA and is associated with specific speech disorders in this variant (e.g., anomia and difficulties in repeating long phrases and sentences), which results from the dysfunction of short-term phonological memory [27]. Asymmetric atrophy of the hippocampus and amygdala is also characteristic of LV-PPA and most likely develops because of underlying Alzheimer’s degeneration. The spread of atrophy to more posterior regions with the involvement of the parietal and occipital lobes and the cerebellum may be explained by the same process.
The loss of the volume of the left temporal pole, the same as that of the IFG, insular lobes, and lateral prefrontal cortex, was also reported in LV-PPA, generally, at more advanced stages. It appears to reflect the involvement of other speech areas and correlate with the onset of symptoms more typical of other PPA variants, such as difficulty understanding individual words [16].
Atrophy of the precentral gyrus, OFC, and medial regions of the frontal lobe is less common even in the advanced stages of LV-PPA. As mentioned above, OFC involvement may be associated with emotional and behavioral disorders. The loss of the cingulate cortex volume, in turn, has been repeatedly described in Alzheimer’s disease and may correlate with the development of nonlinguistic cognitive deficits. Severe degeneration of the precentral gyrus is of the greatest interest; despite earlier reports of such changes, they are usually not one of the most significant areas of atrophy and are observed only in the long-term follow-up [3]. None of the patients from the LV-PPA group had any clinical signs of motor cortex involvement at the time of the examination; therefore, atrophy of this area is most probably secondary, not contributing significantly to disease pathogenesis.
Contrary to our expectations, no degenerations of the inferior parietal lobule, supramarginal and angular gyri, and posterior regions of STG were observed in the LV-PPA group, although it is considered the most pathognomonic for this variant and is included in the diagnostic criteria. This fact and the more diffuse focal nature of atrophy identified in our study in LV-PPA may be accounted for by the relatively small sample size and its pathomorphological heterogeneity. Although Alzheimer-type degeneration prevails in LV-PPA and is observed in 85%–100% of cases [28, 29], it was only present in one-third of our sample. Previously, atrophy patterns in PPA may vary depending on the underlying pathomorphological process, which may have affected the results of this study [30]. Our results demonstrate that the atrophy of the posterior perisylvian regions is not key for the development of the clinical presentations of LV-PPA and indirectly emphasizes a greater contribution of MTG to disease pathogenesis.
Study limitations
This study has several limitations. As mentioned earlier, the sample sizes of the LV-PPA and SV-PPA groups were small. The genetic and pathomorphological heterogeneities of the study groups may be considered a relative limitation. Although it improves the representation of the PPA patient population, it may affect the VBM results because every genetic and pathomorphological variant could have its specific patterns. In addition, we did not perform a correlation analysis as part of the study, comparing the identified atrophy with clinical manifestations, which prevents us from making an unambiguous conclusion on the clinical significance of the changes detected and on the role of the newly identified areas of atrophy on PPA pathogenesis. These limitations should be considered when planning further research on the subject.
CONCLUSION
This study identified patterns of GM atrophy characteristic of each PPA variant using the VBM. The results are consistent with current knowledge of the functional anatomy of speech functions and social behavior. Our findings are partly consistent with those of previous studies conducted in other countries. However, several distinctive features were identified, which require further validation in larger samples.
ADDITIONAL INFORMATION
Funding source. This research was funded by Russian Science Foundation, grant number 23-25-00483.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. D.R. Akhmadullina participated in clinical and neuroimaging data collection, data analysis and interpretation and original draft preparation; R.N. Konovalov conceptualized and supervised the study, performed data analysis and interpretation, reviewed and edited the manuscript; Yu.A. Shpilyukova collected clinical data, reviewed and edited the manuscript; E.Yu. Fedotova planned research design, supervised the study, reviewed and edited the manuscript.
Sobre autores
Diliara Akhmadullina
Research Center of Neurology
Autor responsável pela correspondência
Email: akhmadullinadr1@gmail.com
ORCID ID: 0000-0001-6491-2891
Código SPIN: 5721-8567
Rússia, Moscow
Rodion Konovalov
Research Center of Neurology
Email: krn_74@mail.ru
ORCID ID: 0000-0001-5539-245X
Código SPIN: 2515-7673
Scopus Author ID: 23497502900
Researcher ID: B-6834-2012
MD, Cand. Sci. (Med.)
Rússia, MoscowYulia Shpilyukova
Research Center of Neurology
Email: jshpilyukova@gmail.com
ORCID ID: 0000-0001-7214-583X
Código SPIN: 7502-8984
MD, Cand. Sci. (Med.)
Rússia, MoscowEkaterina Fedotova
Research Center of Neurology
Email: ekfedotova@gmail.com
ORCID ID: 0000-0001-8070-7644
Código SPIN: 3466-2212
MD, Dr. Sci. (Med.)
Rússia, MoscowBibliografia
- Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011; 76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6
- Bisenius S, Neumann J, Schroeter ML. Validating new diagnostic imaging criteria for primary progressive aphasia via anatomical likelihood estimation meta-analyses. European Journal of Neurology. 2016;23(4):704–712. doi: 10.1111/ene.12902
- Lombardi J, Mayer B, Semler E, et al. Quantifying progression in primary progressive aphasia with structural neuroimaging. Alzheimer’s & Dementia. 2021;17(10):1595–1609. doi: 10.1002/alz.12323
- Chapman CA, Polyakova M, Mueller K, et al. Structural correlates of language processing in primary progressive aphasia. Brain Communications. 2023;5(2). doi: 10.1093/braincomms/fcad076
- Canu E, Agosta F, Battistella G, et al. Speech production differences in English and Italian speakers with nonfluent variant PPA. Neurology. 2020;94(10):e1062–e1072. doi: 10.1212/WNL.0000000000008879
- Akhmadullina D, Konovalov R, Shpilyukova Y, et al. Brain atrophy patterns in patients with frontotemporal dementia: voxel-based morphometry. Bulletin of Russian State Medical University. 2020;(6):84–89. doi: 10.24075/brsmu.2020.075
- Lampe L, Huppertz HJ, Anderl-Straub S, et al. Multiclass prediction of different dementia syndromes based on multi-centric volumetric MRI imaging. NeuroImage: Clinical. 2023;37:103320. doi: 10.1016/j.nicl.2023.103320
- Staffaroni AM, Ljubenkov PA, Kornak J, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019;142(2):443–459. doi: 10.1093/brain/awy319
- zenodo.org [Internet]. spunt/bspmview: BSPMVIEW v.20161108 (Version 20161108). Zenodo. [cited 26 July 2023]. Available from: https://zenodo.org/badge/latestdoi/21612/spunt/bspmview doi: 10.5281/zenodo.168074
- Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825
- Tetzloff KA, Utianski RL, Duffy JR, et al. Quantitative analysis of agrammatism in agrammatic primary progressive aphasia and dominant apraxia of speech. Journal of Speech, Language, and Hearing Research. 2018;61(9):2337–2346. doi: 10.1044/2018_JSLHR-L-17-0474
- Whitwell JL, Duffy JR, Strand EA, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain and Language. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005
- Mandelli ML, Vitali P, Santos M, et al. Two insular regions are differentially involved in behavioral variant FTD and nonfluent/agrammatic variant PPA. Cortex. 2016;74:149–157. doi: 10.1016/j.cortex.2015.10.012
- Cordella C, Quimby M, Touroutoglou A, et al. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92(17):e1992–e2004. doi: 10.1212/WNL.0000000000007367
- Breining BL, Faria AV, Tippett DC, et al. Association of Regional Atrophy With Naming Decline in Primary Progressive Aphasia. Neurology. 2023;100(6):e582–e594. doi: 10.1212/WNL.0000000000201491
- Rogalski E, Cobia D, Harrison TM, et al. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c
- Samra K, MacDougall AM, Bouzigues A, et al. Genetic forms of primary progressive aphasia within the GENetic Frontotemporal dementia Initiative (GENFI) cohort: comparison with sporadic primary progressive aphasia. Brain Communications. 2023;5(2). doi: 10.1093/braincomms/fcad036
- Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. The Lancet Neurology. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2
- McKenna MC, Li Hi Shing S, Murad A, et al. Focal thalamus pathology in frontotemporal dementia: Phenotype-associated thalamic profiles. Journal of the Neurological Sciences. 2022;436:120221. doi: 10.1016/j.jns.2022.120221
- Ziegler W, Ackermann H. Subcortical Contributions to Motor Speech: Phylogenetic, Developmental, Clinical. Trends in Neurosciences. 2017;40(8):458–468. doi: 10.1016/j.tins.2017.06.005
- Migliaccio R, Boutet C, Valabregue R, et al. The Brain Network of Naming: A Lesson from Primary Progressive Aphasia. PLOS ONE. 2016;11(2):e0148707. doi: 10.1371/journal.pone.0148707
- Wisse LEM, Ungrady MB, Ittyerah R, et al. Cross-sectional and longitudinal medial temporal lobe subregional atrophy patterns in semantic variant primary progressive aphasia. Neurobiology of Aging. 2021;98:231–241. doi: 10.1016/j.neurobiolaging.2020.11.012
- Fittipaldi S, Ibanez A, Baez S, et al. More than words: Social cognition across variants of primary progressive aphasia. Neuroscience & Biobehavioral Reviews. 2019;100:263–284. doi: 10.1016/j.neubiorev.2019.02.020
- Brown JA, Deng J, Neuhaus J, et al. Patient-Tailored, Connectivity-Based Forecasts of Spreading Brain Atrophy. Neuron. 2019;104(5):856–868.e5. doi: 10.1016/j.neuron.2019.08.037
- Collins JA, Montal V, Hochberg D, et al. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain. 2017;140(2):457–471. doi: 10.1093/brain/aww313
- Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139(3):986–998. doi: 10.1093/brain/awv387
- Henry ML, Wilson SM, Babiak MC, et al. Phonological Processing in Primary Progressive Aphasia. Journal of Cognitive Neuroscience. 2016;28(2):210–222. doi: 10.1162/jocn_a_00901
- Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, Anatomical, and Pathological Features in the Three Variants of Primary Progressive Aphasia: A Review. Frontiers in Neurology. 2018;9. doi: 10.3389/fneur.2018.00692
- Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Annals of Neurology. 2018;84(5):729–740. doi: 10.1002/ana.25333
- Preiß D, Billette OV, Schneider A, et al. The atrophy pattern in Alzheimer-related PPA is more widespread than that of the frontotemporal lobar degeneration associated variants. NeuroImage: Clinical. 2019;24:101994. doi: 10.1016/j.nicl.2019.101994
Arquivos suplementares