Диагностическая и экономическая оценка применения комплексного алгоритма искусственного интеллекта, направленного на выявление десяти патологических находок по данным компьютерной томографии органов грудной клетки
- Авторы: Чернина В.Ю.1, Беляев М.Г.1, Силин А.Ю.2, Аветисов И.О.2, Пятницкий И.А.1,3, Петраш Е.А.1,4, Басова М.В.1, Синицын В.Е.5,6, Омельяновский В.В.7,8,9, Гомболевский В.А.1,10
-
Учреждения:
- АЙРА Лабс
- Клинический госпиталь на Яузе
- Техасский университет в Остине
- Национальный медицинский исследовательский центр онкологии имени Н.Н. Блохина
- Московский государственный университет имени М.В. Ломоносова
- Научно-практический клинический центр диагностики и телемедицинских технологий
- Центр экспертизы и контроля качества медицинской помощи
- Российская медицинская академия непрерывного профессионального образования
- Научно-исследовательский финансовый институт
- Институт искусственного интеллекта
- Выпуск: Том 4, № 2 (2023)
- Страницы: 105-132
- Раздел: Оригинальные исследования
- Статья получена: 07.04.2023
- Статья одобрена: 22.05.2023
- Статья опубликована: 12.07.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/321963
- DOI: https://doi.org/10.17816/DD321963
- ID: 321963
Цитировать
Аннотация
Обоснование. Технологии искусственного интеллекта призваны помогать в решении проблемы пропуска находок при лучевых исследованиях. Важным вопросом является оценка экономической пользы от внедрения технологий искусственного интеллекта.
Цель ― оценить частоту выявления патологических находок и экономический потенциал применения комплексного искусственного интеллекта для компьютерной томографии органов грудной клетки, валидированного экспертами, по сравнению с рентгенологами без доступа к технологиям в условиях частного медицинского центра.
Материалы и методы. Проведено обсервационное одноцентровое ретроспективное исследование. В исследование включались компьютерные томограммы органов грудной клетки без внутривенного контрастирования, выполненные в ООО «Клинический госпиталь на Яузе» (Москва) в период с 01.06.2022 по 31.07.2022. Компьютерные томограммы обработаны комплексным алгоритмом искусственного интеллекта для десяти патологий: инфильтративные изменения в лёгких, характерные для вирусной пневмонии (COVID-19 в условиях пандемии); лёгочные узлы; свободная жидкость в плевральных полостях; эмфизема лёгких; увеличение диаметра грудной аорты; увеличение диаметра ствола лёгочной артерии; коронарный кальциноз; оценка толщины надпочечников; оценка высоты и плотности тел позвонков. Два эксперта анализировали компьютерные томограммы и сравнивали результаты с анализом искусственного интеллекта. Для всех находок, выявленных и не выявленных врачами клиники, определили дальнейшую маршрутизацию в соответствии с клиническими рекомендациями. Для каждого пациента была рассчитана стоимость неоказанных медицинских услуг по прайс-листу клиники.
Результаты. Итоговую группу составили 160 компьютерных томограмм органов грудной клетки с описаниями. С помощью искусственного интеллекта выявлено 90 (56%) исследований с патологиями, из них в 81 (51%) протоколе была пропущена хотя бы одна патология. Общая стоимость неоказанных медицинских услуг «второго этапа» для всех патологий от 81 пациента была оценена в 2 847 760 руб. (37 250,99 долларов или 256 217,95 китайских юаней). Стоимость неоказанных медицинских услуг только для тех патологий, которые пропущены врачами, но выявлены искусственным интеллектом, составила 2 065 360 руб. (27 016,57 долларов или 185 824,05 китайских юаней).
Заключение. Применение искусственного интеллекта для анализа данных компьютерной томографии органов грудной клетки в качестве помощника рентгенолога позволяет существенно уменьшить число случаев пропуска патологий. Использование искусственного интеллекта может принести в 3,6 раза больше стоимости за медицинские услуги по сравнению со стандартной моделью работы рентгенологов без применения таких технологий, и, таким образом, быть рентабельным для применения в условиях частного медицинского центра.
Ключевые слова
Полный текст
Список сокращений и аббревиатур
Датасет (англ. Data set) ― набор данных, коллекция логических записей
ДИ ― доверительный интервал
ИИ ― искусственный интеллект
КТ ― компьютерная томография
МКБ-10 ― Международная статистическая классификация болезней и проблем, связанных со здоровьем, Десятого пересмотра
МРТ ― магнитно-резонансная томография
СНМУ ― стоимость неоказанных медицинских услуг
ОБОСНОВАНИЕ
По данным Всемирной организации здравоохранения, большая часть смертей связана с сердечно-сосудистыми, онкологическими, инфекционными заболеваниями и болезнями лёгких1. На основании крупных рандомизированных исследований по скринингу рака лёгкого, использование низкодозной компьютерной томографии органов грудной клетки (КТ ОГК) среди бессимптомных пациентов групп риска привело к снижению смертности не только от рака лёгкого, но и от всех причин на 6,7% в рамках Национального скринингового исследования лёгких (National Lung Screening Trial, NLST, США) и на 39% с 5-го по 10-й год наблюдения в мультицентрическом исследовании лёгких (Multicentric Italian Lung Detection, MILD, Италия) благодаря выявлению случайных клинически значимых находок, а также лечению и профилактике соответствующих заболеваний [1, 2].
Программы скрининга рака лёгкого признаны экономически эффективными в группах пациентов с высоким риском заболевания. Этот эффект различается в зависимости от систем здравоохранения в разных странах [3]. При этом отмечается, что в этих программах разница между смертностью от рака лёгкого и общей смертностью существенна. Так, в одном из исследований по скринингу рака лёгкого 77,1% пациентов умерли не от самого заболевания, а от других причин, таких как сердечно-сосудистые патологии, болезни лёгких, другие опухоли, инфекционные заболевания и т.д. [1]. Сфокусировавшись на задаче поиска рака лёгкого, врач-рентгенолог может пропустить патологические находки, связанные с другими заболеваниями. Так, указывалось, что при скрининге рака лёгкого 58% клинически значимых находок не отражаются в текстах протоколов врачей-рентгенологов [4].
Во время пандемии COVID-19 программы скрининга рака лёгкого были приостановлены, так как томографы требовались для проведения массовых КТ ОГК в целях диагностики коронавирусной инфекции. Отмечалось, что у половины пациентов, прошедших КТ ОГК, выявлялись случайные находки, у 1/4 они были клинически значимыми [5]. Объём данных, получаемый при КТ ОГК, позволяет диагностировать заболевания не только лёгких, но и других органов и систем [6–8]. Из-за дефицита медицинских кадров, профессионального выгорания, эффекта пандемии и увеличения нагрузки на врача создаётся угроза пропуска клинически значимых находок.
Самые большие надежды на решение этой проблемы возлагаются на технологии искусственного интеллекта (ИИ), поэтому важным вопросом является оценка экономической пользы от применения инновации. Среди сервисов ИИ для здравоохранения наибольшее количество продуктов было создано для лучевой диагностики: их в несколько раз больше, чем во всех остальных медицинских специальностях, вместе взятых2. В Российской Федерации (РФ) самым большим проектом, использующим ИИ для лучевой диагностики, является эксперимент по использованию инновационных технологий в области компьютерного зрения для анализа медицинских изображений и дальнейшего применения в системе здравоохранения города Москвы (далее ― Московский эксперимент), в рамках которого было обработано более 7,5 млн лучевых исследований, включая рентгенографические, маммографические и компьютерно-томографические3 [9].
Обобщая вышесказанное, применение алгоритмов ИИ, направленных на поиск одной патологии, имеет ограниченное значение для практической работы по борьбе с заболеваниями, являющимися основной причиной смертности. Учитывая потребность одномоментного выявления нескольких видов патологий с помощью ИИ, появились первые программные продукты, предлагающие комплексный анализ КТ ОГК, прошедшие все этапы тестирования и разрешённые для применения в проспективном режиме в 153 медицинских организациях города Москвы4. Одним из таких продуктов является комплексный ИИ Multi-IRA от компании «АЙРА Лабс», который может одновременно осуществлять поиск десяти патологических признаков различных заболеваний на КТ [10–13], а именно:
1) инфильтративных изменений в лёгких, характерных для вирусной пневмонии (COVID-19 в условиях пандемии; U07 по Международной классификации болезней 10-го пересмотра, МКБ-10), с оценкой процента поражения лёгких;
2) лёгочных узлов с оценкой размера, объёма и плотности для выявления злокачественных новообразований в лёгких (C34 по МКБ-10);
3) свободной жидкости (выпота) в плевральных полостях (J94 по МКБ-10);
4) эмфиземы лёгких как проявления хронической обструктивной болезни лёгких (J44 по МКБ-10);
5) определения диаметра грудной аорты для поиска её дилатации и аневризм (I70 и I71 по МКБ-10);
6) определения диаметра ствола лёгочной артерии для поиска причин возможной лёгочной гипертензии (I27 по МКБ-10);
7) анализ выраженности коронарного кальциноза по индексу Agatston для оценки тяжести коронарного атеросклероза и риска ишемической болезни сердца (I20–I25 по МКБ-10);
8) оценки толщины надпочечников для поиска образований и гиперплазии (C74 по МКБ-10);
9) высоты тел позвонков для диагностики компрессионных переломов (M80–M85 по МКБ-10);
10) анализ плотности тел позвонков для поиска признаков остеопороза (M80–M85 по МКБ-10).
Цель исследования ― оценить частоту выявления значимых патологических находок и экономический потенциал применения технологий комплексного ИИ при анализе КТ ОГК, валидированного врачами-рентгенологами, по сравнению с врачами-рентгенологами без доступа к ИИ в условиях частного медицинского центра.
МАТЕРИАЛЫ И МЕТОДЫ
Дизайн исследования
Проведено обсервационное одноцентровое ретроспективное исследование. Информированное согласие от пациентов не требовалось. Статья подготовлена в соответствии с контрольным списком CHEERS 2022, предназначенным для экономической оценки исследований в медицине5 [14]. Был разработан план экономического анализа для частного медицинского центра в виде оценки потенциальной дополнительной стоимости неоказанных медицинских услуг (СНМУ), которые должны быть предоставлены пациентам согласно клиническим рекомендациям и лучшим практикам доказательной медицины на основании патологических находок. СНМУ может принести применение комплексного ИИ сверх того, что приносят врачи-рентгенологи, за счёт дальнейших диагностических действий для уточнения природы и тяжести выявленных при КТ находок.
В рамках данного исследования оценка стоимости лечения не проводилась.
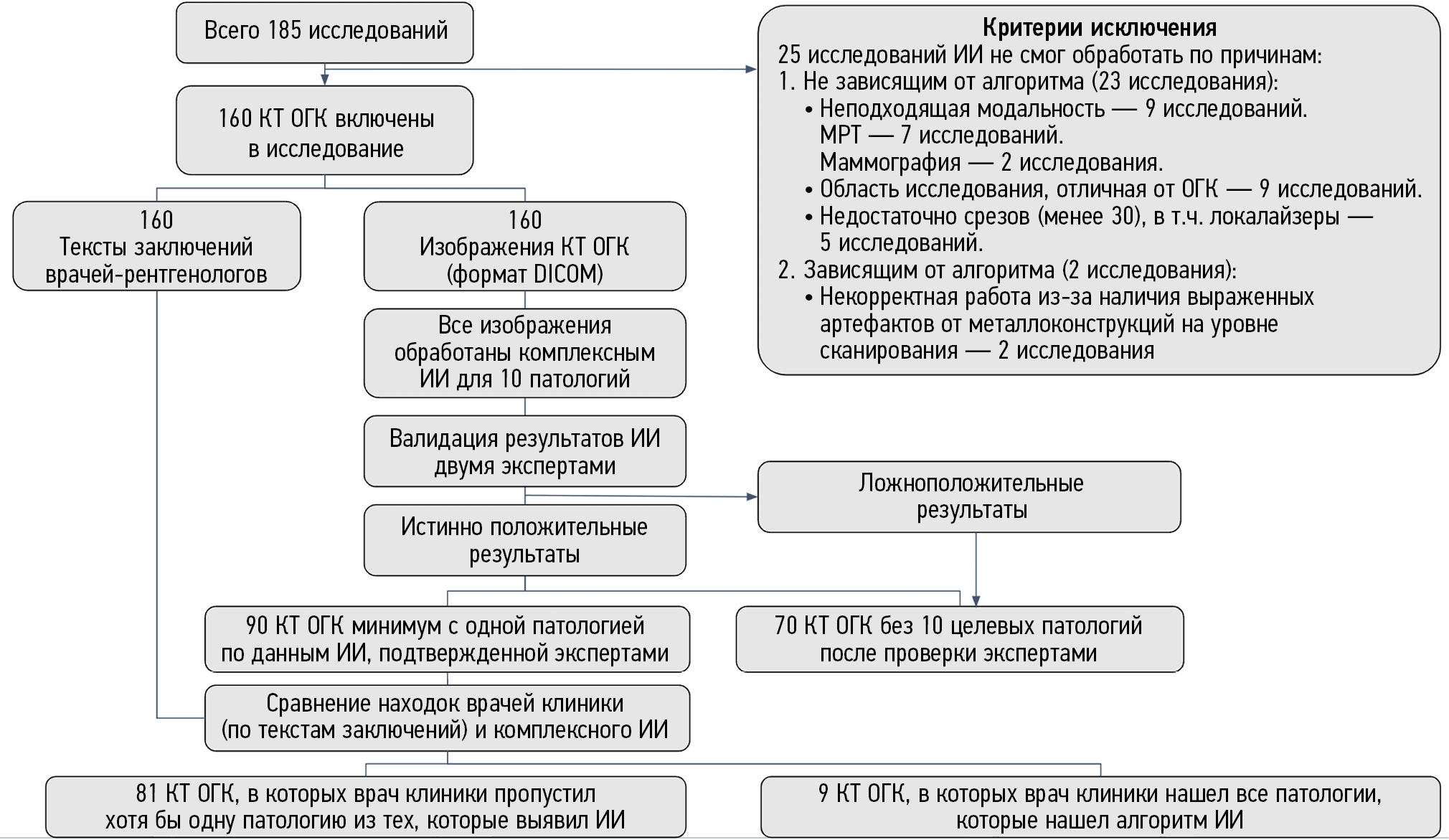
Схема проведения исследования представлена на рис. 1.
Рис. 1. Дизайн исследования.
Критерии соответствия
Для формирования группы исследования были использованы критерии включения и исключения.
Критерии включения: КТ ОГК мужчин и женщин, обратившихся за медицинской помощью в медицинскую организацию, оказывающую первичную санитарную и специализированную медицинскую помощь взрослому населению города Москвы; КТ ОГК проведены и интерпретированы врачами-рентгенологами в период с 01.06.2022 по 31.07.2022; КТ ОГК проведены без внутривенного контрастирования; возраст пациентов старше 18 лет; наличие изображений КТ-исследований в формате DICOM и наличие текстов протоколов описаний исследований от врачей-рентгенологов медицинской организации; первичное посещение пациентом медицинской организации.
Критерии исключения: возраст старше 85 лет; предыдущее КТ ОГК выполнялось в пределах одного года; ИИ не смог обработать исследование по причинам, не зависящим от алгоритма (например, неподходящая модальность, область исследования, отличная от ОГК, недостаточное число срезов ― менее 30); ИИ не смог обработать исследование по причинам, зависящим от особенностей работы самого алгоритма (например, некорректная работа из-за наличия выраженных артефактов от металлоконструкций на уровне сканирования).
Условия проведения
КТ-исследования проводились в многопрофильной частной медицинской организации ООО «Клинический госпиталь на Яузе», оказывающей первичную медико-санитарную и специализированную медицинскую помощь взрослому населению Москвы.
Продолжительность исследования
Исследование проведено на данных КТ, выполненных в период с 01.06.2022 по 31.07.2022. Ретроспективный анализ с помощью ИИ и проверка результатов экспертами проводились в период с 01.10.2022 по 30.11.2022.
Описание исследования
КТ ОГК были проведены без внутривенного контрастирования на компьютерном томографе Philips Ingenuity CT. Томограф выполнял 128 срезов за один оборот трубки. Протокол сканирования ОГК выполнялся в соответствии со стандартными рекомендациями производителя оборудования и национальными рекомендациями. Результаты КТ предоставлялись врачам и ИИ в двух сериях: выполненные при реконструкции с мягкотканным кернелем (60 HU ― центр окна, 360 HU ― ширина окна) и лёгочным кернелем (-500 HU ― центр окна, 1500 HU ― ширина окна). Толщина срезов равнялась 1,0 мм. Использовались алгоритмы итеративной модельной реконструкции (iterative model reconstruction, IMR), направленной на повышение качества изображения (снижения шума) и снижение дозы лучевой нагрузки на пациента.
Все включённые КТ-исследования были обработаны с помощью программы комплексного ИИ Multi-IRA компании «АЙРА Лабс», интегрированной в электронный архив (picture archivingand communication system, PACS) клиники. Используемые в данном исследовании алгоритмы ИИ ранее прошли тестирование на специально подготовленных калибровочных датасетах в рамках Московского эксперимента по применению ИИ6.
Критерием возможности использования алгоритма ИИ являлась их точность не ниже площади под ROC-кривой (ROC AUC) 0,81 для каждой патологии, согласно методическим рекомендациям по клиническим испытаниям программного обеспечения на основе интеллектуальных технологий [15]. Величины метрик диагностической точности для алгоритмов ИИ, полученные на независимых от разработчика закрытых датасетах в рамках Московского эксперимента, представлены в табл. 1 [9, 10].
Таблица 1. Величины метрик диагностической точности для комплексного искусственного интеллекта для компьютерной томографии органов грудной клетки, полученные на датасетах в рамках Московского эксперимента
Название алгоритма искусственного интеллекта Multi-IRA для отдельных видов патологий | ROC AUC | Чувствительность | Специфичность | Точность |
COVID-IRA (выявление областей инфильтрации лёгких) | 0,98 | 0,95 | 0,94 | 0,94 |
LungNodule-IRA (выявление узлов в лёгких) | 0,932 | 0,86 | 0,9 | 0,88 |
PleuralEffusion-IRA (выявление плеврального выпота) | 0,999 | 0,98 | 1 | 0,99 |
Aorta-IRA (ОГК) (анализ диаметра грудной аорты) | 0,997 | 0,96 | 1 | 0,98 |
Aorta-IRA (ОГК+ОБП) (анализ диаметра грудной и брюшной орты) | 1 | 0,98 | 1 | 0,99 |
PulmTrunk-IRA (анализ диаметра ствола лёгочной артерии) | 1 | 1 | 0,98 | 0,99 |
Agatston-IRA (анализ кальциевого индекса по Agatson) | 0,986 | 0,96 | 0,96 | 0,96 |
Genant-IRA (анализ высоты тел позвонков) | 0,995 | 1 | 0,98 | 0,99 |
Emphysema-IRA (выявление эмфиземы) | 0,989 | 0,94 | 0,98 | 0,96 |
Adrenal-IRA (оценка толщины надпочечников для поиска образований и гиперплазии) | 0,96 | 1 | 0,96 | 0,98 |
Основной исход исследования
Для всех находок, выявленных и пропущенных врачами медицинской организации, были определены «вторые этапы» (консультации профильных специалистов и различные виды клинического, инструментального и лабораторного дообследования), т.е. дальнейшая маршрутизация пациента в соответствии с действующими клиническими рекомендациями для каждой патологии (доп. материалы табл.10).
Затем для каждого пациента была рассчитана СНМУ по прайс-листу клиники, которая определялась исходя из невыполненных, согласно клиническим рекомендациям, необходимых медицинских услуг ввиду пропущенной патологии. Дополнительно был проведён расчёт СНМУ от пропусков значимых видов патологии в соответствии с табл. 2 [16–31]. Пропусками у врачей считалось отсутствие информации о патологической находке в финализированных текстах описаний КТ в электронной медицинской информационной системе медицинской организации при наличии такой информации по данным истинно положительных результатов (ground truth) после пересмотра данных КТ врачами-экспертами в сочетании с анализом ИИ. Истинно положительными считались находки, выявленные алгоритмом ИИ, которые были подтверждены двумя врачами-экспертами при анализе изображений КТ.
Таблица 2. Критерии патологических находок и пропусков
Патология | Критерии патологии | Критерии пропуска значимой патологии | Критерии пропуска незначимой патологии |
Патология | Критерии патологии | Критерии пропуска значимой патологии | Критерии пропуска незначимой патологии |
Лёгочные узлы | Хотя бы один солидный или субсолидный (измеряется только солидный компонент) узел со средним размером ≥6 мм (объём ≥100 мм3) [16] | Все находки данной патологии, соответствующие описанным критериям, считаются значимыми, кроме перифиссуральных узлов. Узлы по ходу плевры являются доброкачественными изменениями и не требуют дальнейшего наблюдения | |
Инфильтративные изменения в лёгких, характерные для вирусной пневмонии (COVID-19) в условиях пандемии) | 1. Инфильтрация лёгочной паренхимы по типу матового стекла с обеих сторон, преимущественно периферической локализации, с наличием или без инфильтрации лёгочной паренхимы по типу консолидации с положительным признаком воздушной бронхограммы. 2. Инфильтрация лёгочной паренхимы по типу булыжной мостовой (утолщение междолькового интерстиция на фоне «матового стекла») с обеих сторон, преимущественно периферической локализации, с наличием или без инфильтрации лёгочной паренхимы по типу консолидации с положительным признаком воздушной бронхограммы [17, 18] | Объём поражения лёгких >50% | Объём поражения лёгких <50% |
Эмфизема лёгких | Наличие суммарно в обоих лёгких ≥6% (по объёму) участков (без учёта просвета бронхов) с КТ плотностью ≤-950 HU [19, 20] | Все находки данной патологии, соответствующие описанным критериям, считались значимыми | |
Свободная жидкость (выпот) в плевральных полостях | Наличие в плевральной полости серповидного скопления жидкостного содержимого (выпота) плотностью 0–30 HU в гравитационно зависимых отделах грудной клетки [21] | Максимальная толщина слоя >10 мм | Максимальная толщина слоя <10 мм |
Аневризма / дилатация аорты | 1. Дилатация восходящей части грудной аорты: на нативных изображениях наибольший диаметр восходящей части грудной аорты от 40 до 49 мм включительно в аксиальной плоскости. 2. Аневризма восходящей части грудной аорты: на нативных изображениях наибольший диаметр восходящей части грудной аорты ≥50 мм в аксиальной плоскости. 3. Дилатация нисходящей части грудной аорты: на нативных изображениях наибольший диаметр нисходящей части грудной аорты от 31 до 39 мм в аксиальной плоскости. 4. Аневризма нисходящей части грудной аорты: на нативных изображениях наибольший диаметр нисходящей части грудной аорты ≥40 мм в аксиальной плоскости# [22]. 5. Дилатация брюшного отдела аорты: наибольший диаметр от 25 до 29 мм включительно. 6. Аневризма брюшного отдела аорты: наибольший диаметр ≥30 мм [23, 24] | Аневризма восходящей части (диаметр ≥50 мм) и нисходящей части грудной аорты (диаметр ≥40 мм); аневризма брюшного отдела аорты (диаметр ≥30 мм) | Дилатация восходящей части (диаметр от 40 до 49 мм) и нисходящей части (диаметр от 31 до 39 мм) грудной аорты; дилатация брюшного отдела аорты (диаметр ≥30 мм) |
Дилатация лёгочного ствола | Диаметр лёгочного ствола ≥29 мм## [25] | >29 мм | 29 мм |
Коронарный кальциноз по индексу Agatston | На нативных изображениях кальциевый индекс/индекс Agatston (сумма площадей в проекции коронарных сосудов, умноженных на индивидуальные факторы плотности*) ≥1, либо класс CAC-DRS A1–A3 [26, 27]. *Фактор 1: 130–199 HU Фактор 2: 200–299 HU Фактор 3: 300–399 HU Фактор 4: ≥400 HU | Индекс Agatston >10 | Индекс Agatston от 1 до 10 |
Оценка толщины надпочечников для поиска образований и гиперплазии | Узловые образования тела или ножек надпочечника с диаметром по короткой оси ≥10 мм [28] | Все находки данной патологии, соответствующие описанным критериям, считаются значимыми | |
Оценка плотности губчатого вещества тел позвонков для выявления остеопороза / остеопении | Снижение минеральной плотности костной ткани в телах позвонков в интервале Th11–L3 (оптимально L1–L2) согласно критериям ACR 2018, позиции ISCD 2019 [29] | Плотность <+100 HU | Плотность от +100 до +150 HU |
Компрессионные переломы позвонков для выявления остеопороза | Наличие позвонков, имеющих компрессионную деформацию тел ≥25% по полуколичественной шкале Genant, II–III степень [30, 31]. Степень деформации (СД) рассчитывается по формуле: СД = (отношение максимального размера тела позвонка - минимальный/максимальный размер) × 100% | Все находки данной патологии, соответствующие описанным критериям, считались значимыми | |
Дополнительные исходы исследования
Дополнительно для каждого врача-рентгенолога клиники было посчитано количество протоколов со значимыми и незначимыми пропусками, а также проценты ошибочных протоколов.
Анализ в подгруппах
Два эксперта (врачи-рентгенологи с опытом работы 10 и 13 лет соответственно, не являющиеся сотрудниками медицинской организации, данные которой применялись в исследовании), пересмотрели КТ вместе с результатами обработки алгоритмом ИИ для исключения ошибочных срабатываний программы. При противоположных мнениях принималось единое решение после коллегиального обсуждения. В результате такого анализа были отобраны истинно положительные результаты анализа комплексного ИИ, подтверждённые двумя экспертами. Затем находки, предложенные ИИ, были сопоставлены с финализированными текстами протоколов описаний КТ ОГК, написанными врачами-рентгенологами медицинской организации без использования ИИ, и были выявлены случаи пропуска патологий. Все пропуски патологии врачами-рентгенологами медицинской организации были разделены на значимые и незначимые. Критерии значимости патологий оценивались в соответствии с базовыми диагностическими требованиями к результатам работы ИИ, используемыми для проведения Московского эксперимента, согласованными научно-проблемной комиссией ГБУЗ НПКЦ ДиТ ДЗМ, протоколы от 10.12.2021 № 9/2021, от 28.02.2022 № 1/2022, от 06.12.2022 № 7/2022, от 13.01.2023 № 1/2023. Эти требования основаны на клинических рекомендациях и лучших практиках, обоснованных с точки зрения доказательной медицины.
Оценка текстов протоколов по наличию/отсутствию упоминания эпикардиального жира не проводилась в данном исследовании, так как у врачей-рентгенологов медицинской организации не было инструментов для измерения объёмов жировой ткани. Критерии патологий, а также распределение по значимости пропусков представлены в табл. 2.
Этическая экспертиза
Было отправлено уведомление в Независимый этический комитет Московского регионального отделения Российского общества рентгенологов и радиологов о проведении ретроспективного исследования (протокол от 01.03.2023).
Статистический анализ
Для представления результатов были использованы методы описательной статистики с указанием абсолютного числа (n) и доли (%) наблюдений в каждой категории. Сравнение частоты обнаружения патологий разными методами проводилось с помощью Z-теста для пропорций. Полученные для каждой из 9 патологий р-значения корректировались на множественное тестирование (в рамках общей гипотезы об отсутствии статистически значимой разницы между результатами диагностик) поправкой Бонферрони. Анализ финансовых показателей выполнялся с помощью парного t-теста. Уровнем статистической значимости для р считалось значение 0,05. Статистический анализ был проведён с помощью программы R v. 4.1.3.
РЕЗУЛЬТАТЫ
Объекты (участники) исследования
Всего было отобрано 185 исследований (соотношение мужчин/женщин ― 47/53%; возраст от 19 до 83 лет, средний возраст 49,5 лет), соответствующих указанным критериям, из них 25 исследований не были обработаны ИИ по следующим причинам:
1) независящие от ИИ (23 исследования):
- неподходящая модальность (9 исследований: МРТ ― 7; маммография ― 2);
- область исследования, отличная от ОГК (9 исследований);
- недостаточное число срезов (менее 30), в том числе локалайзеры (5 исследований);
2) зависящие от ИИ (2 исследования):
- некорректная работа из-за наличия выраженных артефактов от металлоконструкций на уровне сканирования (2 исследования).
Итоговую группу для анализа составили 160 случаев КТ ОГК с текстами описаний врачей. Дополнительная информация о наличии у пациентов онкологических, сердечно-сосудистых и других хронических заболеваний не собиралась, так как в данную медицинскую организацию пациенты обращались только по полисам обязательного или добровольного медицинского страхования, а также на платной основе к узкопрофильным специалистам. Информация в медицинской информационной системе об установленных диагнозах с высокой вероятностью была неполноценной.
Основные результаты исследования
Была настроена автоматическая анонимизация и передача КТ-исследований от медицинской организации к разработчику комплексного ИИ для КТ ОГК, а также возвращение результатов анализа в медицинскую организацию и экспертам для валидации результатов работы ИИ. Эксперты предоставили список всех расхождений между верифицированными результатами ИИ и текстами протоколов сотрудникам медицинской организации для контроля качества (рис. 2). Апелляций со стороны клиники не последовало. Наибольшее количество клинически значимых пропусков в текстах протоколов выявлено для случаев остеопороза и образований надпочечников (по 14 случаев). Наибольшее количество незначимых пропусков выявлено для расширения аорты (36 случаев) и остеопении (40 случаев). Подробные результаты по количеству находок представлены на рис. 3.
Рис. 2. Результаты исследования по количеству находок, выявленных с/без использования искусственного интеллекта.
Рис. 3. Результаты исследования по количеству находок (ранжированы по количеству значимых пропусков).
С помощью ИИ выявлено 90 (56%) исследований с патологией, из них в 81 (51%) протоколе врачей медицинской организации была пропущена хотя бы одна патология. В 70 исследованиях алгоритм ИИ не выявил патологий. Следует отметить, что в представленных исследованиях могли быть другие патологические находки, которые не входили в программу анализа программы комплексного ИИ в этом исследовании. Сводные данные анализа представлены в табл. 3.
Таблица 3. Сводные данные по количеству пациентов с выявленными и пропущенными патологиями
Параметр | Количество | Число всех случаев, % | Оценка патологии, % |
Всего пациентов | 160 | 100 | - |
Без патологических находок | 70 | 44 | - |
С патологическими находками | 90 | 56 | 100 |
Врач медицинской организации нашёл хотя бы одну патологию из тех, которые выявил искусственный интеллект | 35 | 22 | 39 |
Врач медицинской организации пропустил хотя бы одну патологию из тех, которые выявил искусственный интеллект | 81 | 51 | 90 |
Компьютерно-томографические исследования, в которых патологические изменения нашёл только алгоритм искусственного интеллекта | 55 | 34 | 61 |
В одном КТ-исследовании могло быть несколько патологий, часть из которых нашёл врач из медицинской организации (описал в протоколе), а другую часть нашёл только ИИ (потенциальная польза ИИ).
Детальное представление анализа по СНМУ вследствие пропусков патологии по каждому из 90 пациентов показано на рис. 4.
Рис. 4. Анализ стоимости неоказанных медицинских услуг вследствие пропусков патологий по всему диапазону выполненных компьютерных томографий (КТ).
При сопоставлении двух диагностических подходов (алгоритм с проверкой экспертом против врача медицинской организации без ИИ) статистически значимыми оказались сравнения для следующих патологических находок: аневризмы/дилатации аорты; расширения диаметра ствола лёгочной артерии; оценки коронарного кальциевого индекса, компрессионных переломов позвонков, снижения плотности тел позвонков и утолщения надпочечников (табл. 4).
Таблица 4. Сравнение двух диагностических подходов к поиску анализируемых патологий
Параметр | Количество выявленных случаев | Доля [95% ДИ] | 95% ДИ для разности долей | р Z-тест H0: доли равны (коррекция Бонферрони) |
Признаки COVID-19 по данным компьютерной томографии | ||||
Алгоритм+эксперт | 3 | [-0,008; 0,04] | 0,246 (1) | |
Протокол | 0 | - | - | |
Лёгочные узлы | ||||
Алгоритм+эксперт | 15 | [-0,036; 0,08] | 0,542 (1) | |
Протокол | 11 | - | - | |
Выпот | ||||
Алгоритм+эксперт | 6 | [-0,028; 0,05] | 0,749 (1) | |
Протокол | 4 | - | - | |
Аорта | ||||
Алгоритм+эксперт | 40 | <0,001 (<0,001) | ||
Протокол | 2 | - | - | |
Ствол лёгочной артерии | ||||
Алгоритм+эксперт | 16 | 0,005 (0,042) | ||
Протокол | 3 | - | - | |
Коронарный кальциноз | ||||
Алгоритм+эксперт | 29 | 0,001 (0,01) | ||
Протокол | 9 | - | - | |
Компрессионные переломы позвонков | ||||
Алгоритм+эксперт | 18 | 0,002 (0,015) | ||
Протокол | 3 | - | - | |
Оценка минеральной плотности тел позвонков | ||||
Алгоритм+эксперт | 74 | <0,001 (<0,001) | ||
Протокол | 20 | - | - | |
Утолщения надпочечников | ||||
Алгоритм+эксперт | 26 | <0,001 (0,001) | ||
Протокол | 4 | - | - | |
Предполагаемая СНМУ «второго этапа» для всех пропущенных патологий у 81 пациента суммарно составила 2 847 760 руб. ($37 250,99 или 256 217,95 CNY), на одного пациента ― 17 799 руб. ($232,83 или 1601,41 CNY). СНМУ «второго этапа» только для тех патологий, которые были пропущены в протоколах врачами медицинской организации, но были выявлены ИИ и подтверждены экспертами, суммарно составила 2 065 360 руб. ($27 016,57 или 185 824,05 CNY), на одного пациента ― 12 909 руб. ($168,86 или 1161,45 CNY). Результаты расчёта СНМУ для всех находок представлены в табл. 5.
Таблица 5. Анализ предполагаемой стоимости неоказанных медицинских услуг от всех пропущенных находок на компьютерной томографии
Параметр | Расчёт на всех пациентов пилотного проекта (руб. / доллары США / юани) | Расчёт на одного пациента (руб. / доллары США / юани) | ||||||
Стоимость | ||||||||
Суммарная (все) | Консультации (все) | Дообследования (все) | Follow-up (все) | Суммарная (все) | Консультации (все) | Дообследования (все) | Follow-up (все) | |
Стоимость «второго этапа» диагностики для всех патологий | 2 847 760/ 37 250,99/ 256 217,95 | 463 300/ 6060,34/ 41 683,91 | 2 049 760/ 26 812,51/ 184 420,49 | 334 700/ 4378,15/ 30 113,54 | 17 799/ 232,83/ 1601,41 | 2896/ 37,88/ 260,56 | 12 811/ 167,58/ 1152,63 | 2092/ 27,37/ 188,22 |
Стоимость «второго этапа» только по тем патологиям, которые были пропущены врачом и найдены алгоритмом искусственного интеллекта | 2 065 360/ 27 016,57/ 185 824,05 | 326 800/ 4274,81/ 29 402,77 | 1 519 460/ 19 875,76/ 136 708,47 | 219 100/ 2866/ 19 712,81 | 12 909/ 168,86/ 1161,45 | 2043/ 26,72/ 183,81 | 9497/ 124,23/ 854,46 | 1369/ 17,91/ 123,17 |
Стоимость «второго этапа» только по патологиям, найденным врачами | 782 400/ 10 234,42/ 70 393,9 | 136 500/ 1785,53/ 12 281,14 | 530 300/ 6936,75/ 47 712,02 | 115 600/ 1512,14/ 10 400,73 | 4890/ 63,97/ 439,96 | 853/ 11,16/ 76,75 | 3314/ 43,35/ 298,17 | 723/ 9,46/ 65,05 |
По результатам расчётов, суммарная СНМУ только от значимых пропусков составила 770 855 руб. ($10 083,4 или 69 355,17 CNY), на одного пациента ― 4818 руб. ($63,02 или 433,48 CNY). Результаты анализа представлены в табл. 6.
Таблица 6. Результаты по стоимости неоказанных медицинских услуг от пропуска значимых находок
Параметр | Расчёт на всех пациентов пилотного проекта (руб. / доллары / юани) | Расчёт на одного пациента (руб. / доллары / юани) | ||||||
Стоимость | ||||||||
Суммарная (все) | Консультации (все) | Дообследования (все) | Follow-up (все) | Суммарная (все) | Консультации (все) | Дообследования (все) | Follow-up (все) | |
Стоимость «второго этапа» по отношению к значимым пропускам патологии врачами | 770 855/ 10 083,4/ 69 355,17 | 113 100/ 1479,44/ 10 175,8 | 584 255/ 7642,53/ 52 566,44 | 73 500/ 961,44/ 6612,92 | 4818/ 63,02/ 433,48 | 707/ 9,25/ 63,61 | 3652/ 47,77/ 328,58 | 459/ 6/ 41,3 |
Стоимость «второго этапа» по отношению к найденным врачами патологиям | 782 400/ 10 234,42/ 70 393,9 | 136 500/ 1785,53/ 12 281,14 | 530 300/ 6936,75/ 47 712,02 | 115 600/ 1512,14/ 10 400,73 | 4890/ 63,97/ 439,96 | 853/ 11,16/ 76,75 | 3314/ 43,35/ 298,17 | 723/ 9,46/ 65,05 |
Сравнение стоимостей проводили при помощи парного t-теста с вычислением средней разницы на одного пациента и построения 95% доверительного интервала (ДИ). Таким образом, 160×12 908,5 [160×9833,5; 160×15 983,5] даёт общую СНМУ по популяции анализа со своим ДИ. Результаты представлены в табл. 7.
Таблица 7. Экономическая эффективность
Когорта | Статистика | Стоимость | р (парный t-тест) | ||
Суммарная (все) | Суммарная (находки врачей) | Средняя разница с 95% ДИ | |||
Полная когорта | n | 160 | 160 | 12 908,5 | <0,001 |
Среднее | 17 798,5 | 4890 | |||
СО | 23 304,74 | 11 945 | - | ||
Минимум | 0 | 0 | - | ||
Первый квартиль | 0 | 0 | - | ||
Медиана | 12 635 | 0 | - | ||
Третий квартиль | 27 900 | 0 | - | ||
Максимум | 91 095 | 52 420 | - | ||
% нулевых значений | 46,88 | 79,38 | - | ||
Когорта со всеми обнаруженными находками | n | 81 | 81 | 24 298,4 | <0,001 |
Среднее | 33 503,06 | 9204,71 | [19 701,9; 28 894,8] | ||
СО | 22 262,46 | 15 162,13 | - | ||
Минимум | 3900 | 0 | - | ||
Первый квартиль | 14 395 | 0 | - | ||
Медиана | 27 900 | 0 | - | ||
Третий квартиль | 51 995 | 14 395 | - | ||
Максимум | 91 095 | 52 420 | - | ||
% нулевых значений | - | 61,18 | - | ||
Когорта по значимым находкам | n | 32 | 32 | 17 104,7 | <0,001 |
Среднее | 24 089,22 | 6984,53 | |||
СО | 15 568,37 | 13 799,84 | - | ||
Минимум | 7600 | 0 | - | ||
Первый квартиль | 14 395 | 0 | - | ||
Медиана | 19 700 | 0 | - | ||
Третий квартиль | 27 900 | 4825 | - | ||
Максимум | 69 500 | 52 420 | - | ||
% нулевых значений | - | 71,88 | - | ||
Итоговая экономическая эффективность использования алгоритма ИИ в медицинской организации представлена на рис. 5.
Рис. 5. Спектр стоимости неоказанных медицинских услуг от применения комплексного искусственного интеллекта для компьютерной томографии органов грудной клетки в медицинской организации. ИИ ― искусственный интеллект; КТ ― компьютерная томография; ОГК ― органы грудной клетки; СНМУ ― стоимость неоказанных медицинских услуг.
Пример расчёта стоимости неоказанных медицинских услуг
Врач из медицинской организации верно описал в протоколе расширение ствола лёгочной артерии до 34 мм, повышенный кальциевый индекс по Agatston до 350, снижение плотности позвонков максимально до +90 HU; алгоритм ИИ также нашёл перечисленные патологии. Кроме того, алгоритм ИИ нашёл патологии, которые врач не описал, а именно узел в лёгком диаметром до 10×9 мм, дилатацию грудного отдела аорты до 45 мм, утолщение надпочечника до 14 мм. Пример расчёта СНМУ в таком случае представлен в табл. 8.
Таблица 8. Пример расчёта стоимости неоказанных медицинских услуг по данным одной компьютерной томографии органов грудной клетки
Рекомендации и стоимость ко всем имеющимся у пациентов патологиям | ||||||
Консультации (все) | Дообследования (все) | Наблюдения в динамике (все) | Стоимость | |||
Суммарная (все) | Консультации (все) | Дообследования (все) | Наблюдения в динамике (все) | |||
Консультации кардиолога, эндокринолога | Стресс-ЭКГ, определение активности ЩФ, ОАК, исследование уровня кальция, УЗИ ОБП, КТ-коронарография, стресс-Эхо, исследование гормональной активности опухоли надпочечника, ЭхоКГ, биохимический анализ крови, КТ с внутривенным контрастированием | КТ в динамике, консультация эндокринолога | 91 095 руб./ $1191,6/ 8195,98 CNY | 7800 руб./ $102,03/ 701,78 CNY | 71 795 руб./ $939,14/ 6459,52 CNY | 11 500 руб./ $150,43/ 1034,68 CNY |
Рекомендации и стоимость только к тем патологиям, которые были пропущены врачами и найдены алгоритмом | ||||||
Консультации | Дообследования | Наблюдения в динамике | Стоимость | |||
Суммарная | Консультации | Дообследования | Наблюдения в динамике | |||
- | Стресс-ЭКГ, КТ-коронарография, стресс-Эхо, исследование гормональной активности опухоли надпочечника, ЭхоКГ, КТ с внутривенным контрастированием | КТ в динамике | 65 300 руб./ $854,18/ 5875,16 CNY | 0 | 57 700 руб./ $754,76/ 5191,37 CNY | 7600 руб./ $99,41/ 683,79 CNY |
Рекомендации и стоимость только к патологиям, найденным врачами | ||||||
Консультации | Дообследования | Наблюдения в динамике | Стоимость | |||
Суммарная | Консультации | Дообследования | Наблюдения в динамике | |||
Консультации кардиолога, эндокринолога | Определение активности ЩФ, ОАК, исследование уровня кальция, УЗИ органов брюшной полости, биохимический анализ крови | Консультация эндокринолога | 25 795 руб./ $337,42/ 2320,82 CNY | 7800 руб./ $102,03/ 701,78 CNY | 14 095 руб./ $184,37/ 1268,15 CNY | 3900 руб./ $51,02/ 350,89 CNY |
Примечание. ЩФ ― щелочная фосфатаза; ОАК ― общий анализ крови; УЗИ ОБП ― ультразвуковое исследование органов брюшной полости; КТ ― компьютерная томография; ЭхоКГ ― эхокардиография.
Дополнительные результаты исследования
Итоговые результаты по количеству протоколов со значимыми и незначимыми пропусками, а также проценты ошибочных протоколов представлены в табл. 9. В одном и том же протоколе могли быть как значимые, так и незначимые пропуски патологий. Всего из 160 проанализированных протоколов в 81 были найдены значимые и незначимые пропуски, что составило 50,6% от общего количества КТ. Средний процент протоколов со значимыми пропусками составил 28,1% (max 56,9; min 5), с незначимыми пропусками ― 27,2% (max 74,1; min 5).
Таблица 9. Итоговые результаты по количеству протоколов со значимыми и незначимыми пропусками
№ врача | Общее количество написанных протоколов | Количество протоколов с незначимыми пропусками (%) | Количество протоколов со значимыми пропусками (%) | Общее количество протоколов со значимыми и незначимыми пропусками (%) |
Врач № 2 | 58 | 33 (56,9) | 28 (48,3) | 47 (81) |
Врач № 5 | 23 | 7 (30,4) | 9 (39,1) | 11 (39,1) |
Врач № 3 | 23 | 7 (30,4) | 7 (30,4) | 13 (56,5) |
Врач № 1 | 16 | 2 (12,5) | 3 (18,7) | 5 (31) |
Врач № 6 | 20 | 1 (5) | 1 (5) | 2 (10) |
Врач № 7 | 18 | 1 (5,5) | 1 (5,5) | 2 (11,1) |
Врач № 4 | 2 | 1 (50) | 1 (50) | 1 (50) |
Всего | 160 | 52 (32,5) | 50 (31,2) | 81 (50,6) |
Общее число протоколов статистически значимо увеличивало количество ошибок. Общий стаж работы в рентгенологии (без учёта ординатуры) и торакальной рентгенологии (с учётом ординатуры) уменьшал количество ошибок. Однако эти данные не являются репрезентативными в силу малой выборки врачей и наличия доминантного случая. Подробные данные по стажу врачей представлены в дополнительных материалах (табл. 10, 11).
Примеры работы алгоритма ИИ представлены на рис. 6 и 7.
Рис. 6. Пример работы алгоритма искусственного интеллекта. Пациент Б., 76 лет. Врач корректно выявил двусторонний гидроторакс и эмфизематозные изменения, однако не описал лёгочный узел в правом лёгком. Алгоритм искусственного интеллекта выявил все 3 патологии: гидроторакс оконтурен жёлтой линией, эмфизематозные изменения выделены оранжевым цветом, лёгочный узел обозначен красным квадратом.
Рис. 7. Пример работы алгоритма искусственного интеллекта. Пациент В., 79 лет. Компьютерная томография органов грудной клетки: а ― аксиальный срез: врач и алгоритм корректно выявили лёгочный узел в левом лёгком (обозначен красным квадратом) и коронарный кальциноз (оконтурен оранжевой линией); дополнительно алгоритм обозначил увеличение объёма эпикардиального жира (залито жёлтым цветом, в исследовании данная патология не учитывалась); b ― сагиттальный срез: врач и алгоритм корректно выявили компрессионные переломы тел Th6 и Th9 позвонков, Genant 3 (3 колонны обозначены красными линиями), однако врач в протоколе не указал деформации тел Th5 и Th12 позвонков, Genant 2 (3 колонны обозначены жёлтыми линиями).
Нежелательные явления
В результате исследования нежелательных явлений не отмечалось.
ОБСУЖДЕНИЕ
Резюме основного результата исследования
В результате исследования удалось впервые показать предполагаемый экономический эффект, который возможно получить от применения комплексного программного продукта на основе технологии ИИ для анализа данных КТ ОГК. В основе предполагаемого экономического эффекта лежит оценка СНМУ.
Медицинские услуги необходимо оказывать пациентам согласно актуальным клиническим рекомендациям. Суммарная СНМУ «второго этапа» необходимой диагностики только для тех патологий, которые были пропущены врачами, но выявлены ИИ, составила чуть более 2 млн руб., или в 3,6 раз больше стоимости за медицинские услуги, которые могли быть оказаны в клинике в соответствии только с находками, выявленными врачами, у 160 пациентов по данным КТ ОГК. По результатам использованной модели расчёта, СНМУ только от значимых пропусков составила чуть более 770 тыс. руб. согласно прайс-листу медицинской организации, или на 98% больше, чем стоимость медицинских услуг, которую могли принести врачи медицинской организации благодаря выявлению случайных находок.
Дополнительно показана возможность использования комплексного программного продукта на основе технологии ИИ для аудита протоколов описаний КТ.
Обсуждение основного результата исследования
Анализ социально-экономического бремени пандемии COVID-19 может служить примером значимых социальных и экономических последствий того или иного массового заболевания для системы здравоохранения РФ и общества в целом, что акцентирует внимание не только на клинической, но и на экономической важности инвестиций в разработку стратегий борьбы с заболеваниями [32]. По оценкам экспертов, социально-экономическое бремя COVID-19 в РФ за 2020 год составило около 5,4 трлн рублей (5% от номинального объёма ВВП в 2020 году), что соответствует 2486,30 YLL (англ. years of life lost ― сумма потерянных лет жизни из-за преждевременной смертности населения) среди мужчин и 1378,22 YLL среди женщин [32]. Экономическое бремя неинфекционных заболеваний в РФ за тот же год составило 4 трлн рублей, при этом ущерб от хронических заболеваний сопоставим с бюджетом всего здравоохранения РФ, а средства, которые можно высвободить за счёт эффективной профилактики, могли бы стать колоссальным дополнительным ресурсом для развития страны7.
В доступной литературе мы не нашли исследований, оценивающих влияние комплексных систем ИИ для анализа КТ ОГК на экономические аспекты работы медицинской организации.
P.J. Pickhardt с соавт. [33] построили модель экономической и клинической эффективности скрининга на основе комплексного ИИ с использованием КТ для органов брюшной полости. Используя ожидаемую распространённость заболеваний, вероятности перехода между состояниями здоровья, связанные с ними затраты на здравоохранение и эффективность лечения трёх заболеваний (сердечно-сосудистые заболевания, остеопороз и саркопения), были оценены 3 взаимоисключающие модели скрининга: (1) игнорирование результатов («не лечить»; никаких вмешательств независимо от результатов КТ); (2) универсальная терапия статинами («лечить всех» для профилактики сердечно-сосудистых заболеваний без учёта результатов КТ); (3) оппортунистический скрининг сердечно-сосудистых заболеваний, остеопороза и саркопении с помощью КТ брюшной полости, выявленных на основе ИИ (целевое лечение лиц групп риска). Для базовых сценариев для групп 55-летних мужчин и женщин, смоделированных в течение 10 лет, оппортунистический скрининг на основе КТ с помощью ИИ был экономичной и более эффективной клинической стратегией, чем подходы «игнорировать» и «лечить всех». Авторы резюмируют, что оппортунистический скрининг на основе КТ с помощью ИИ представляется высокорентабельной и клинически эффективной стратегией при широком наборе входных допущений и в большинстве сценариев обеспечивает экономию средств. Однако в сравнении с нашим исследованием реально работающий комплексный ИИ представлен не был. Кроме того, в нашей работе использовался комплексный ИИ, направленный на 10 целевых патологий, по сравнению с тремя в упомянутой работе. В связи с этим есть надежда на повышение потенциала экономической эффективности применения ИИ в сочетании с его положительным влиянием на диагностику.
В единичных публикациях изучался экономический эффект подобных программ при использовании комплексного подхода для нескольких патологий без применения систем ИИ. Так, для Нидерландов было рассчитано, что комплексный низкодозный КТ-скрининг трёх заболеваний, таких как рак лёгкого, хроническая обструктивная болезнь лёгких и сердечно-сосудистые заболевания у лиц в возрасте от 50 до 75 лет, может быть экономически эффективен, если он будет стоить менее 971 евро на одного обследованного человека [34]. В пятилетнем исследовании по данным почти 7,5 тыс. низкодозных КТ было определено, что внелёгочные злокачественные опухоли, случайно выявленные во время скрининга рака лёгкого, имели ранние стадии и благоприятный исход, а дополнительные обследования требовали гораздо меньших затрат, чем в случаях с более поздними стадиями [35]. По мнению авторов, проведённый анализ затрат на дополнительные диагностические и лечебные мероприятия, связанные с выявленными внелёгочными изменениями в ходе низкодозного КТ-скрининга рака лёгкого, является одним из основных этапов доказательства экономической эффективности подобных мероприятий. Такой подход (хотя и ограниченный скрининг рака лёгкого с помощью КТ ОГК) позволяет предлагать применение именно комплексного ИИ для повышения диагностической и экономической эффективности исследований.
В нашем исследовании также был использован комплексный ИИ для поиска 10 патологий. Такие анализы, называемые анализом запаса, оказались полезными для принятия обоснованных решений о дальнейших исследованиях [36–38]. Они являются предпочтительными во время диагностического вмешательства и разработки доказательств для оптимизации сбора данных и более точной оценки долгосрочного экономического воздействия на здоровье, когда становится доступен большой объём клинических данных.
До пандемии COVID-19 алгоритмы ИИ использовались для выявления рентгенологических симптомов с целью обнаружения заболеваний, классификации, оптимизации изображений, снижения дозы лучевой нагрузки и улучшения рабочего процесса [39]. Медицинские исследования эффективности применения ИИ делают такие программы более понятными, безопасными, эффективными и интегрированными в рабочие процессы врачей [40]. В настоящее время идут исследования в рамках проекта IMALife, изучающие снижение смертности не только от рака лёгкого, но и от последствий эмфиземы (биомаркер хронической обструктивной болезни лёгких) и коронарного кальциноза (биомаркер атеросклероза) [41].
Оценка эффективности применения ИИ до настоящего времени встречалась только в алгоритмах ИИ, предназначенных для поиска лишь одной целевой патологии. В исследовании S. Ziegelmayer и колл. [42] в базовом сценарии КТ+ИИ был показан отрицательный коэффициент дополнительной эффективности затрат (incremental cost-effectiveness ratio, ICER) по сравнению с КТ, демонстрируя более низкие затраты и более высокую эффективность. Пороговый анализ показал, что ICER оставался отрицательным до порога в $68 для поддержки применения ИИ. Так, использование моносистемы анализа данных низкодозной КТ с помощью ИИ для скрининга рака лёгкого является разумной диагностической стратегией с точки зрения экономической эффективности.
Постоянно растущий объём лучевых исследований создаёт дополнительную нагрузку на врача-рентгенолога [43]. Избыточная нагрузка может увеличить вероятность ошибок и поставить под угрозу качество оказания медицинской помощи [44]. Система аудита с ретроспективным двойным пересмотром исследований широко распространена в лучевой диагностике. Наиболее известной является система RADPEER system Американского общества рентгенологов [45]. Однако, согласно исследованию P.M. Lauritzen с соавт. [46], двойное чтение 1/3 исследований, выполненных в их учреждении, занимает около 20–25% рабочего времени врачей. Использование алгоритмов ИИ может значительно сократить время на пересмотр исследований и увеличить объём и качество аудита, при этом важным условием является минимальное количество ложноположительных ошибок алгоритмов. Применение ИИ также влияло на качество работы врачей-рентгенологов в виде изменения соотношения степени тяжести поражений лёгких при подозрении на COVID-19 в сторону сокращения удельного веса тяжёлой и критической оценки степени тяжести поражения лёгких [47].
В нашем исследовании мы также продемонстрировали возможность использования комплексного ИИ для ауди-та описаний исследований и выявили более 28% протоколов со значимыми и 27% протоколов с незначимыми пропусками патологий. Следует отметить, что для всех КТ-исследований врачи-рентгенологи медицинской организации в своих протоколах дали ответы на основные диагностические задачи, ради которых пациенты были направлены на сканирование. Врачи медицинской организации, на базе которой проводилось исследование, не имели на всех рабочих станциях инструментов для быстрого измерения индекса Agatston, а измерение плотности тел позвонков не входило в стандарт описания исследований в данном отделении лучевой диагностики. Кроме того, было показано, что средний процент ошибок сопоставим между врачами, поэтому увольняя 1–2 врачей с худшими результатами описаний по данным аудитов, клиника не решит проблему пропуска патологий.
В данной работе при расчёте экономического эффекта не учитывалась стоимость затрат на ИИ, а также стоимость работы, выполненной экспертами по валидации результатов ИИ. Стоимость этих расходов вариабельна в зависимости от количества алгоритмов, уровня привлекаемых экспертов и других факторов. Экономически эффективным будет любой тариф на услугу по применению комплексного ИИ при условии, что суммарные затраты будут ниже, чем получение прибыли медицинской организацией, благодаря применению ИИ (рис. 8). Однако такой анализ выходит за рамки текущего исследования.
Рис. 8. Потенциал стоимости неоказанных медицинских услуг из-за отсутствия применения комплексного искусственного интеллекта для компьютерной томографии органов грудной клетки в медицинской организации с учётом расходов на использование искусственного интеллекта (ИИ).
Ограничения исследования
Данное исследование является пилотным и имеет ряд ограничений.
Так, исследование имеет ретроспективный дизайн и оценивает максимальный потенциал СНМУ частной медицинской организации, обоснованной рекомендациями, следующими за патологическими находками. На практике не все пациенты склонны прислушиваться к врачам и выполнять обоснованные рекомендации, тем более в тех случаях, когда речь идёт о платных дообследованиях и консультациях. Кроме того, конверсия в каждой клинике имеет свои особенности, выходящие за пределы дизайна текущего исследования.
В цели настоящего исследования входила лишь оценка стоимости «второго этапа» без стоимости «третьего» и последующих этапов, т.е. не учитывалась стоимость лечения и реабилитации. Однако почти для каждой патологии, которую способен выявить используемый комплексный ИИ, стоимость лечения значительно превосходит стоимость «второго этапа». В каждой медицинской организации есть множество факторов, влияющих на качество работы врачей. Показатель качества работы врачей (количество пропусков клинически значимых рентгенологических находок) может быть вариабелен в зависимости от стажа врачей-рентгенологов, количества исследований в день, времени суток, дня недели и многих других дополнительных показателей, способных повлиять на знания, внимательность и готовность врача-рентгенолога внести в протокол все патологические находки и обоснованные рекомендации для «второго этапа».
В нашей работе не оценивался потенциал ложноотрицательных находок ИИ, так как используемый комплексный ИИ валидирован при независимых тестированиях на закрытых датасетах Московского эксперимента, и выбранные настройки ИИ были признаны приемлемыми и откалиброванными для работы.
В цели данного исследования не входила оценка экономического эффекта на уровне городского и федерального здравоохранения, однако каждая медицинская организация в РФ не лишена возможности проведения платных медицинских услуг населению, обоснованных с точки зрения принципов доказательной медицины.
В нашем исследовании не изучалась комплаентность пациентов по приглашениям на основе ретроспективного анализа, при этом выявленные находки тесно связаны с временными интервалами между проведением КТ-исследования и «вторым этапом».
ЗАКЛЮЧЕНИЕ
Применение комплексного ИИ для анализа данных КТ ОГК в качестве помощника врача-рентгенолога с целью диагностики 10 распространённых и важных видов патологических находок приводит к улучшению их выявления. Важно, что потенциал СНМУ для «второго этапа» при таком подходе в 3,6 раза больше по сравнению со стандартной моделью работы врачей-рентгенологов без помощи ИИ.
Оппортунистический скрининг нескольких заболеваний требует детального изучения сопутствующих заболеваний для определения оптимальной целевой группы диагностического вмешательства с помощью комплексного ИИ. Высока вероятность того, что применение комплексного ИИ для КТ ОГК будет рентабельным, так как при таком подходе выявляется большое количество значимых патологических изменений, требующих дополнительных медицинских услуг.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Авторы заявляют об отсутствии внешнего финансирования при проведении исследования.
Конфликт интересов. Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов. Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией). Наибольший вклад распределён следующим образом: В.Ю. Чернина ― поиск публикаций по теме статьи, формирование набора данных, написание текста рукописи; М.Г. Беляев ― экспертная оценка информации, редактирование текста рукописи; А.Ю. Силин ― концепция исследования; И.А. Пятницкий ― редактирование текста рукописи; перевод текста на английский язык; И.О. Аветисов, Е.А. Петраш ― редактирование текста рукописи; М.В. Басова ― формирование набора данных, обработка результатов исследования; В.Е. Синицын ― экспертная оценка информации, редактирование текста рукописи; В.В. Омельяновский ― экспертная оценка информации; В.А. Гомболевский ― концепция исследования, экспертная оценка информации, написание текста рукописи, утверждение финальной версии рукописи.
Благодарности. Коллектив авторов выражает благодарность Писову Максиму Евгеньевичу, техническому директору компании «АЙРА Лабс», и команде разработчиков (Проскурову В.А., Самойленко А.И., Борзову А.С., Бухараеву А.Н., Гончарову М.Ю., Широких Б.Н., Курмукову А.И., Начинкину И.А., Телепову А.Ю., Шимоволос С.А., Донсковой М.А., Самохину В.Ю., Шевцову А.Е., Яушев Ф., Ткаченко С.М., Захарову А.А., Сапарову Т.Н., Филипенко В.К., Томинину В.Д., Томинину Я.Д., Самойленко А.И., Малеванному В.М., Леонову А.Ю., Ногиной Д.С., Базаровой А.И., Мараховскому К.В., Белкову А.С., Васину А.А., Бережной Д.С., Мусихину М.М.); Дуговой Марие Николаевне, медицинскому директору, и команде медицинских экспертов (Ильичевой Д.В., Севрюкову Д.Д., Щипахиной Я.А., Любимой Ю.О., Алешиной О.О., Цыбульской Ю.А.); Ламзину Михаилу Сергеевичу, операционному директору, Гареевой Регине Рустемовне, продакт-менеджеру компании, и Паниной Елене Вячеславовне, заместителю директора по развитию бизнеса.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. The authors confirm that their authorship complies with the international ICMJE criteria (all authors contributed significantly to developing the concept, research, and preparation of the article and read and approved the final version before publication).
Chernina V.Yu. — search for publications on the article’s topic, formation of a data set, writing the text of the manuscript; Belyaev M.G. — expert evaluation of the data, manuscript’s text editing; Silin A. Yu. — the concept of the study; Pyatnitskiy I.A. — manuscript’s text editing; English text translation; Avetisov I.O., Petrash E.A. — manuscript’s text editing; Basova M.V. — formation of a data set, processing of research results; Sinitsyn V.E. — expert evaluation of the data, manuscript’s text editing; Omelyanovsky V.V. — expert evaluation data; Gombolevsky V.A. — the concept of the study, expert evaluation of data, manuscript’s text writing, approval of the final version.
Acknowledgments. The authors’ team would like to gratefully acknowledge Maksim E. Pisov, Technical Director of IRA Labs, and the development team (Proskurov V.A., Samoilenko A.I., Borzov A.S., Bukharaev A.N., Goncharov M.Y., Shirokikh B.N., Kurmukova A.I., Nachinkin I.A., Telepov A.Y., Shimovolos S.A., Donskova M.A., Samokhin V.Y., Shevtsova A.E., Yaushev F., Tkachenko S.M, Zakharov A.A., Saparov T.N., Filipenko V.K. Tomin V.D., Tomin J.D., Samoilenko A.I., Malevannu V.M., Leonov A. Yu, Nogova D.S., Bazarova A.I., Marakhovsky K.V., Belkov A.S., Vasin A.A., Berezhnaya D.S., Musikhin M.M.); Maria N. Dugova, Medical Director, and the team of medical experts (Ilyicheva D.V., Sevrukov D.D., Shchipakhina Y.A, Lyubimaya Y.O., Aleshina O.O., Tsybulskaya Y.A.); Mikhail S. Lamzin, Chief Operating Officer; Regina R. Gareeva, Product Manager, and Elena V. Panina, Deputy Director for Business Development.
1 who.int [интернет]. 10 ведущих причин смерти в мире [цит.: 09.12.2020]. Режим доступа: https://www.who.int/ru/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2 IQVIA [интернет]. FDA Publishes Approved List of AI/ML-enabled Medical Devices [cite 2021 Oct 29]. Michaela Miller, Technology & Analytics Practice Lead, U.S. MedTech, IQVIA. Режим доступа: https://www.iqvia.com/locations/united-states/blogs/2021/10/fda-publishes-approved-list-of-ai-ml-enabled-medical-devices.
3 Центр диагностики и телемедицины [интернет]. Эксперимент (https://mosmed.ai/ai/); Наборы данных (https://mosmed.ai/datasets/). Дата обращения: 17.03.2023.
4 Центр диагностики и телемедицины [интернет]. ООО «АЙРА Лабс» Chest-IRA. Режим доступа: https://mosmed.ai/service_catalog/chestira/.
5 EQUATOR Network [интернет]. Enhancing the QUAlity and Transparency Of health Research. Режим доступа: https://www.equator-network.org/reporting-guidelines/cheers/.
6 Центр диагностики и телемедицины [интернет]. ООО «АЙРА Лабс»: Chest-IRA (https://mosmed.ai/service_catalog/chestira/); Adrenal-IRA Abd (https://mosmed.ai/service_catalog/adrenalira/); Aorta-IRA Abd (https://mosmed.ai/service_catalog/aortaira/); Genant-IRA Abd (https://mosmed.ai/service_catalog/genant-ira/). Дата обращения: 17.03.2023.
# Рабочая группа по диагностике и лечению заболеваний аорты Европейского общества кардиологов (ESC). Рекомендации ESC по диагностике и лечению заболеваний аорты, 2014. Режим доступа: https://scardio.ru/content/Guidelines/Recom%20po%20aorte%207_rkj_15.pdf.
## Рабочая группа по диагностике и лечению легочной гипертензии Европейского общества кардиологов (ESC) и Европейского общества пульмонологов (ERS). Рекомендации ESC/ERS по диагностике и лечению легочной гипертензии, 2015. Режим доступа: https://scardio.ru/content/Guidelines/ESC%20_L_hypert_2015.pdf.
7 Медвестник [интернет]. Экономическое бремя неинфекционных заболеваний в России выросло за два года на триллион рублей [цит.: 04.06.2021]. Режим доступа: https://medvestnik.ru/content/news/Ekonomicheskoe-bremya-neinfekcionnyh-zabolevanii-v-Rossii-vyroslo-za-dva-goda-na-trillion-rublei.html.
ДОПОЛНИТЕЛЬНЫЕ МАТЕРИАЛЫ
Таблица 10. Рекомендации по дополнительным методам обследования
Патология | Клинические рекомендации | Консультации / дообследования /наблюдения в динамике |
Патология | Клинические рекомендации | Консультации / дообследования /наблюдения в динамике |
Лёгочные узлы | Гомболевский В.А., Блохин И.А., Лайпан А.Ш., и др. Методические рекомендации по скринингу рака лёгкого. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 56. Москва: ГБУЗ НПКЦ ДиТ ДЗМ, 2020. 57 с. | Консультации: онколог. Дообследования: биопсия, бронхоскопия под КТ/УЗИ контролем, цитология, анализ крови (ВИЧ, сифилис, гепатит), ФВД, спирометрия, ОАК, ЭКГ, ПЭТ/КТ, МРТ головного мозга с внутривенным контрастированием. Наблюдения в динамике: в зависимости от количества и размеров лёгочных узлов рекомендована КТ ОГК через 3–6 мес |
Инфильтративные изменения в лёгких, характерные для вирусной пневмонии (COVID-19 в условиях пандемии) | Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Министерство здравоохранения Российской Федерации. Версия 17 (14.12.2022). Режим доступа: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/061/254/original/%D0%92%D0%9C%D0%A0_COVID-19_V17.pdf?1671088207 | Консультации: терапевт. Дообследования: пульсоксиметрия; ЭКГ; выявление РНК SARS-CoV-2 с применением метода амплификации нуклеиновых кислот; выявление антигенов SARS-CoV-2 c применением иммунохроматографических методов; ОАК с определением уровня эритроцитов, гемоглобина, гематокрита, лейкоцитов, тромбоцитов, лейкоцитарной формулы; биохимический анализ крови (мочевина, креатинин, электролиты, глюкоза, АЛТ, АСТ, билирубин, альбумин, лактат, лактатдегидрогеназа; дополнительно могут быть исследованы тропонин как маркер повреждения миокарда и ферритин как белок острой фазы воспаления, дающий информацию о тяжести течения заболевания и прогнозе), СРБ; гормональное исследование (прокальцитонин, NT-proBNP); коагулограмма (в объёме активированное частичное тромбопластиновое время, протромбиновое время, протромбиновое отношение и/или % протромбина по Квику, фибриноген, D-димер количественным методом). Наблюдения в динамике: через 8 нед после выписки рекомендуется посещение врача и проведение инструментальных исследований (по показаниям): рентгенографии ОГК*, спирографии*, измерения насыщения крови кислородом (сатурация) в покое и при нагрузке (возможно проведение теста 6-минутной ходьбы с определением сатурации до и после теста)*, ЭхоКГ, других методов исследования (диффузионный тест, газы артериальной крови и др.) * При выявлении патологии ― проведение КТ лёгких |
Эмфизема лёгких | Эмфизема лёгких. Клинические рекомендации (утв. Министерством здравоохранения РФ, 2021). Режим доступа: https://www.garant.ru/products/ipo/prime/doc/402775957/ | Консультации: пульмонолог/терапевт. Дообследования: анализ газов крови. Пациентам с подозрением на наследственный дефицит А1АТ рекомендуется определение активности А1АТ в крови, спирометрия с бронходилатационным тестом, бодиплетизмография и исследование диффузионной способности лёгких. Всем пациентам с клиническими признаками дефицита А1АТ и/или сниженной концентрации А1АТ в сыворотке крови рекомендуется определение фенотипа и генотипа. Наблюдения в динамике: всем пациентам с дефицитом А1АТ рекомендуется ежегодное биохимическое общетерапевтическое исследование крови с определением уровня альбумина в крови, уровня общего билирубина в крови, активности АСТ в крови, активности АЛТ в крови, активности ГГТ в крови, уровня тромбоцитов в крови. Пациентам с лёгочной формой дефицита А1АТ вначале рекомендуется проводить спирометрию каждые 6–12 мес для исключения быстрого прогрессирования заболевания, после чего частота исследований может быть снижена. Пациентам с дефицитом А1АТ рекомендуется ежегодно проводить УЗИ ОБП для исключения проявления болезни со стороны печени |
Свободная жидкость (выпот) в плевральных полостях | Маскелл Н., Бутланд Р. Рекомендации Британского торакального общества (BTS) по обследованию взрослых больных с односторонним плевральным выпотом // Пульмонология. 2006. № 2. С. 13–26. doi: 10.18093/0869-0189-2006-2-13-26 | Консультации: пульмонолог. Если выявлен туберкулёз ― консультация фтизиатра, если злокачественное поражение ― консультация онколога. Дообследования: рассмотреть необходимость аспирации плевральной жидкости (ASAP ― «как можно скорее»); цитологическое исследование; определение белка; ЛДГ, pH; окраска по Граму; посев и определение чувствительности; окраска на кислотоустойчивые палочки; посев на микобактерии туберкулёза. Далее, если необходимо, КТ ОГК с внутривенным контрастированием; биопсия плевры. Если причина после всех исследований не найдена ― рассмотреть вопрос о торакоскопии |
Аневризма/дилатация аорты | Клинические рекомендации. Рекомендации по диагностике и лечению заболеваний аорты (2017) // Кардиология и сердечно-сосудистая хирургия. 2018. № 1. С. 7–67 | Консультации: кардиолог/сосудистый хирург. Дообследования: КТ-аортография с внутривенным контрастированием. Наблюдения в динамике: трансторакальная ЭхоКГ, УЗИ ОБП |
Дилатация ствола лёгочной артерии | Клинические рекомендации. Лёгочная гипертензия, в том числе хроническая тромбоэмболическая лёгочная гипертензия, 2020 (03.06.2021). Утверждены Минздравом РФ. Режим доступа: http://disuria.ru/_ld/10/1026_kr20I27MZ.pdf | Консультации: кардиолог. Дообследования: ЭхоКГ; ЭКГ; газы крови; сцинтиграфия лёгких перфузионная в сочетании со сцинтиграфией лёгких вентиляционной; ОАК с оценкой уровня гемоглобина и гематокрита, количества эритроцитов, лейкоцитов, тромбоцитов, СОЭ; биохимический анализ крови (исследование уровня креатинина, натрия, калия, глюкозы, общего белка, СРБ, АСТ, АЛТ, общего билирубина, мочевой кислоты); определение содержания антител к кардиолипину, фосфолипидам, бета-2-гликопротеину в крови при подозрении на хроническую тромбоэмболическую лёгочную гипертензию для выявления факторов риска; определение содержания антител к антигенам ядра клетки и ДНК для исключения ассоциации с системными заболеваниями соединительной ткани; исследование уровня NT-proBNP; определение антител классов M, G к ВИЧ-1 в крови, антител классов M, G к ВИЧ-2 в крови; определение антигена ВГВ, антител к ВГС, антител к бледной трепонеме; общий (клинический) анализ мочи; оценка функции щитовидной железы (исследование уровня свободного трийодтиронина, свободного тироксина и тиреотропного гормона); УЗИ ОБП (комплексное) для исключения патологии печени и/или портальной гипертензии |
Коронарный кальциноз | Клинические рекомендации. Стабильная ишемическая болезнь сердца, 2020 (Одобрено Научно-практическим советом Минздрава РФ). Режим доступа: https://cr.minzdrav.gov.ru/schema/155_1 | Консультации: кардиолог. Дообследования: ОАК (клинический) развёрнутый с измерением уровня гемоглобина, числа эритроцитов и лейкоцитов; исследование уровня креатинина в крови и оценка состояния функции почек по расчётной СКФ или клиренсу креатинина; биохимический анализ крови, включая исследование уровней общего холестерина крови, холестерина липопротеидов низкой плотности и триглицеридов; исследование уровня NT-proBNP в крови; ЭКГ в покое; амбулаторное мониторирование ЭКГ; ЭхоКГ; МРТ сердца может быть применено при получении неинформативных результатов при ЭхоКГ (в т.ч. с введением контраста); дуплексное сканирование экстракраниальных отделов сонных артерий для выявления атеросклеротических бляшек; КТ-коронарография; стресс-ЭКГ/стресс-ЭхоКГ/стресс-МРТ/ОФЭКТ. Наблюдения в динамике: при повторных исследованиях у всех пациентов с диагнозом стабильной ишемической болезни сердца рекомендуется проводить ежегодный контроль ОАК (клинического) развёрнутого, анализа крови биохимического общетерапевтического, анализа крови по оценке нарушений липидного обмена биохимического; исследование уровня креатинина в крови; исследование уровня глюкозы в крови натощак с целью своевременной коррекции терапии при необходимости |
Оценка толщины надпочечников для поиска образований и гиперплазии | Клинические рекомендации. Рак коры надпочечника (Адренокортикальный рак), 2020 (Одобрено Научно-практическим советом Минздрава РФ). Режим доступа: https://cr.minzdrav.gov.ru/schema/341_1 | Консультации: эндокринолог, онколог. Дообследования: исследование гормональной активности опухоли надпочечника (кортизол, АКТГ, определение свободных метанефринов плазмы или фракционированных метанефринов суточной мочи; определение уровня альдостерона, ренина плазмы и калия сыворотки), КТ ОБП с внутривенным контрастированием (при наличии противопоказаний к выполнению КТ с контрастным усилением провести МРТ органов брюшной полости и забрюшинного пространства). При выявлении образования надпочечника с неопределённым КТ-фенотипом и отсутствии гормональной гиперсекреции рекомендовано рассмотреть ситуацию в консилиуме, при этом возможны три варианта решений: (1) проведение дополнительных визуализирующих исследований (ПЭТ/КТ с фтордезоксиглюкозой); (2) динамическое наблюдение с повторной КТ без контраста (или МРТ) через 3–6 мес; (3) хирургическое лечение. Наблюдения в динамике: при образованиях с неопределённым КТ-фенотипом с целью динамической оценки размеров опухоли (если на основании первичной диагностики было выбрано наблюдение) рекомендована повторная КТ/МРТ через 3–6 мес. Хирургическое лечение показано при увеличении размеров образования на 20% (или увеличение максимального диаметра >5 мм) за короткий период наблюдения |
Оценка плотности губчатого вещества тел позвонков для выявления остеопороза/ остеопении | Клинические рекомендации. Остеопороз, 2021 (Одобрено Научно-практическим советом Минздрава РФ). Режим доступа: https://cr.minzdrav.gov.ru/recomend/87_4 | Консультации: эндокринолог. Дообследования: ОАК (клинический); исследование уровня биохимических параметров крови (общего кальция, креатинина с подсчётом СКФ, неорганического фосфора, определение активности ЩФ, глюкозы); определение С-концевого телопептида в крови, и/или исследование уровня N-терминального пропептида проколлагена 1-го типа (P1NP) в крови (маркера резорбции при назначении антирезорбтивной терапии и костеобразования при назначении анаболической терапии соответственно) пациентам, получающим лечение остеопороза самостоятельно, исходно и через 3 мес от начала терапии с целью ранней оценки эффективности лечения и приверженности к терапии; проведение двухэнергетической рентгеноденситометрии (DXA) поясничного отдела позвоночника и проксимального отдела бедренной кости; трёхмерное измерение максимального потребления кислорода, производимое при количественной КТ (опционально) |
Компрессионные переломы позвонков для выявления остеопороза | Клинические рекомендации. Патологические переломы, осложняющие остеопороз, 2022 (Одобрено Научно-практическим советом Минздрава РФ). Режим доступа: https://cr.minzdrav.gov.ru/recomend/614_2 | Консультации: эндокринолог. Дообследования: ОАК (клинический); общий (клинический) анализ мочи; анализ крови биохимический общетерапевтический с исследованием кальция общего и/или ионизированного, неорганического фосфора, общей ЩФ, креатинина крови с подсчётом СКФ); исследование уровня паратиреоидного гормона в крови, кальция и фосфора суточной мочи; двухэнергетическая рентгеноденситометрия. При наличии низкоэнергетического перелома тела позвонка у лиц старше 50 лет требуется исследование крови и мочи на парапротеины и М-градиент для исключения миеломной болезни |
Примечание. КТ ― компьютерная томография; УЗИ ― ультразвуковое исследование; ВИЧ ― вирус иммунодефицита человека; ФВД ― функция внешнего дыхания; ОАК ― общий анализ крови; ЭКГ ― электрокардиограмма; ПЭТ/КТ ― позитронно-эмиссионная томография, совмещённая с рентгеновской компьютерной томографией; МРТ ― магнитно-резонансная томография; ОГК ― органы грудной клетки; АЛТ ― аланинаминотрансфераза; АСТ ― аспартатаминотрансфераза; СРБ ― С-реактивный белок; NT-proBNP ― мозговой натрий-уретический пептид; ЭхоКГ ― эхокардиография; А1АТ ― альфа-1-антитрипсин; ГГТ ― гамма-глютамилтрансфераза; ОБП ― органы брюшной полости; ЛДГ ― лактатдегидрогеназа; СОЭ ― скорость оседания эритроцитов; ВГВ ― вирус гепатита В; ВГС ― вирус гепатита С; СКФ ― скорость клубочковой фильтрации; ОФЭКТ ― однофотонная эмиссионная компьютерная томография; АКТГ ― адренокортикотропный гормон; ЩФ ― щелочная фосфатаза.
Таблица 11. Стаж работы врачей
Врач | Стаж работы, лет | Объём ставки | Наличие учёной степени | ||
в рентгенологии (с учётом ординатуры) | в торакальной рентгенологии (без учёта ординатуры) | в КТ | |||
Врач № 1 | 15 | 13 | 13 | Частичная | Да |
Врач № 2 | 17 | 5 | 5 | Частичная | Нет |
Врач № 3 | 7 | 5 | 5 | Полная | Нет |
Врач № 4 | 16 | 14 | 14 | Частичная | Нет |
Врач № 5 | 7 | 5 | 5 | Частичная | Нет |
Врач № 6 | 5 | 3 | 3 | Частичная | Да |
Врач № 7 | 14 | 10 | 10 | Частичная | Нет |
Обобщённая линейная модель
Обобщённая линейная модель предполагает, что каждое наблюдение Yi линейно зависит от значений переменных Xip, p=1,2,...m:
Yi=bi0+b1Xi1+b2Xi2+...+bmXim+εi,
где переменные X могут быть как категориальными, учитывающими группы, классы, категории, так и непрерывными. В обобщённой линейной модели оцениваются коэффициенты {bj, j=0,1,2,...,m} для модели зависимости параметра Y от факторов {Xj, j=1,2,...,m}. Для этих коэффициентов определяется их статистическая значимость (вычисление р-значений для проверки гипотез Hj0: bj=0, j=0,1,2,...,m), которая показывает значимость влияния соответствующих факторов на целевой параметр.
В нашем случае некоторые параметры имеют логарифмическое нормальное распределение, поэтому к ним было применено логарифмическое преобразование. В итоге модель имеет вид:
Y=log (Total number of protocols with critical and non-critical errors)
X1=All protocols
X2=log (Work experience in thoracic radiology (excluding residency))
X3=log (Length of experience in CT)
и категориальный параметр X4=Academic degree.
Результаты оценки коэффициентов обобщённой линейной модели и их статистическая значимость приведены в таблице:
Можно сделать вывод, что общее число протоколов статистически значимо увеличивает количество ошибок. Общий стаж работы в рентгенологии и торакальной рентгенологии уменьшает количество ошибок, однако эти данные не являются репрезентативными в силу малой выборки врачей и наличия доминантного случая.
Частично похожие результаты (с точностью до статистической значимости и влияния одного из видов опыта) отражаются и в корреляционном анализе:
- ρ (log (Total number of protocols with critical and non-critical errors), All protocols) = 0,88 с 95% ДИ [0, 39; 0, 98] и р-значением 0,008 (корреляция статистически значима);
- ρ (log (Total number of protocols with critical and non-critical errors), log (Work experience in thoracic radiology (excluding residency)) = -0,45 с 95% ДИ [-0,9; 0,46] и р-значением 0,31 (корреляция статистически незначима);
- ρ (log (Total number of protocols with critical and non-critical errors), log (Work experience in thoracic radiology (excluding residency)) = -0,27 с 95% ДИ [-0,85; 0,61] и р-значением 0,56 (корреляция статистически незначима).
Однако при отсутствии статистической значимости корреляций говорить о каких-то тенденциях не представляется возможным.
Estimate | Std..Error | t.value | Pr...t.. | |
(Intercept) | 0,132922 | 0,352989 | 0,37656 | 0,742697 |
bd_lr$”All protocols” | 0,037246 | 0,006111 | 6,094863 | 0,025879 |
log(bd_lr$”Work experience in thoracic radiology/r/n(excluding residency)/r/n”) | -7,96969 | 1,009013 | -7,8985 | 0,015654 |
factor(bd_lr$”Academic degree”)нет | -0,92975 | 0,229399 | -4,05297 | 0,055828 |
Об авторах
Валерия Юрьевна Чернина
АЙРА Лабс
Email: v.chernina@ira-labs.com
ORCID iD: 0000-0002-0302-293X
SPIN-код: 8896-8051
Scopus Author ID: 57210638679
ResearcherId: AAF-1215-2020
Россия, Москва
Михаил Геннадьевич Беляев
АЙРА Лабс
Email: belyaevmichel@gmail.com
ORCID iD: 0000-0001-9906-6453
SPIN-код: 2406-1772
канд. физ.-мат. наук, профессор
Россия, МоскваАнтон Юрьевич Силин
Клинический госпиталь на Яузе
Email: silin@yamed.ru
ORCID iD: 0000-0003-4952-2347
SPIN-код: 4411-8745
Россия, Москва
Иван Олегович Аветисов
Клинический госпиталь на Яузе
Email: avetisov@yamed.ru
ORCID iD: 0009-0007-3550-7556
Россия, Москва
Илья Аркадьевич Пятницкий
АЙРА Лабс; Техасский университет в Остине
Email: i.pyatnitskiy@ira-labs.com
ORCID iD: 0000-0002-2827-1473
SPIN-код: 6150-4961
Россия, Москва; Остин, США
Екатерина Александровна Петраш
АЙРА Лабс; Национальный медицинский исследовательский центр онкологии имени Н.Н. Блохина
Email: e.a.petrash@gmail.com
ORCID iD: 0000-0001-6572-5369
SPIN-код: 6910-8890
канд. мед. наук
Россия, Москва; МоскваМария Васильевна Басова
АЙРА Лабс
Email: m.basova@ira-labs.com
ORCID iD: 0009-0000-3325-8452
Россия, Москва
Валентин Евгеньевич Синицын
Московский государственный университет имени М.В. Ломоносова; Научно-практический клинический центр диагностики и телемедицинских технологий
Email: vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-код: 8449-6590
д-р мед. наук, профессор
Россия, Москва; МоскваВиталий Владимирович Омельяновский
Центр экспертизы и контроля качества медицинской помощи; Российская медицинская академия непрерывного профессионального образования; Научно-исследовательский финансовый институт
Email: vvo@rosmedex.ru
ORCID iD: 0000-0003-1581-0703
SPIN-код: 1776-4270
д-р мед. наук, профессор
Россия, Москва; Москва; МоскваВиктор Александрович Гомболевский
АЙРА Лабс; Институт искусственного интеллекта
Автор, ответственный за переписку.
Email: gombolevskii@gmail.com
ORCID iD: 0000-0003-1816-1315
SPIN-код: 6810-3279
канд. мед. наук
Россия, Москва; МоскваСписок литературы
- Aberle D.R., Adams A.M., Berg C.D., et al.; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening // N Engl J Med. 2011. Vol. 365, N 5. P. 395–409. doi: 10.1056/NEJMoa1102873
- Pastorino U., Silva M., Sestini S., et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: New confirmation of lung cancer screening efficacy // Ann Oncol. 2019. Vol. 30, N 7. P. 1162–1169. doi: 10.1093/annonc/mdz117
- Grover H., King W., Bhattarai N., et al. Systematic review of the cost-effectiveness of screening for lung cancer with low dose computed tomography // Lung Cancer. 2022. N 170. P. 20–33. doi: 10.1016/j.lungcan.2022.05.005
- Николаев А.Е., Гомболевский В.А., Гончар А.П., и др. Случайные находки при скрининге рака легкого методом низкодозной компьютерной томографии // Туберкулез и болезни лёгких. 2018. Т. 96, № 11. С. 60–67. doi: 10.21292/2075-1230-2018-96-11-60-67
- Kilsdonk I.D., de Roos M.P., Bresser P., et al. Frequency and spectrum of incidental findings when using chest CT as a primary triage tool for COVID-19 // Eur J Radiol Open. 2021. N 8. P. 100366. doi: 10.1016/j.ejro.2021.100366
- Бельцевич Д.Г., Мельниченко Г.А., Кузнецов Н.С., и др. Клинические рекомендации Российской ассоциации эндокринологов по дифференциальной диагностике инциденталом надпочечников // Эндокринная хирургия. 2016. Т. 10, № 4. С. 31–42. doi: 10.14341/serg2016431-42
- Эмфизема легких. Клинические рекомендации (утв. Министерством здравоохранения РФ, 2021). Режим доступа: https://www.garant.ru/products/ipo/prime/doc/402775957/. Дата обращения: 17.03.2023.
- Аневризмы грудной и торакоабдоминальной аорты. Клинические рекомендации (утв. Министерством здравоохранения РФ, 2023). Режим доступа: https://www.angiolsurgery.org/library/recommendations/2022/aneurysms_thoracic_thoracoabdominal_aorta/recommendation.pdf. Дата обращения: 17.03.2023.
- Васильев Ю.А., Владзимирский А.В., Арзамасов К.М., и др. Компьютерное зрение в лучевой диагностике: первый этап Московского эксперимента. Монография. 2-е изд., перераб. и доп. / под ред. Ю.А. Васильева, А.В. Владзимирского. Москва: Издательские решения, 2023. 376 с.
- Zakharov A., Pisov M., Bukharaev A., et al. Interpretable vertebral fracture quantification via anchor-free landmarks localization // Med Image Anal. 2023. N 83. P. 102646. doi: 10.1016/j.media.2022.102646
- Goncharov M., Pisov M., Shevtsov A., et al. CT-Based COVID-19 triage: Deep multitask learning improves joint identification and severity quantification // Med Image Anal // 2021. N 71. P. 102054. doi: 10.1016/j.media.2021.102054
- Shirokikh B., Shevtsov A., Dalechina A., et al. Accelerating 3D medical image segmentation by adaptive small-scale target localization // J Imaging. 2021. Vol. 13, N 7. P. 35. doi: 10.3390/jimaging7020035
- Goncharov M., Pisov M., Belyaev M., et al. Quantification of epicardial adipose tissue in low-dose computed tomography images // Lecture Notes in Electrical Engineering. 2022. Vol. 784, N 1. P. 98–107. doi: 10.1007/978-981-16-3880-0_11
- Husereau D., Drummond M., Augustovski F., et al.; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated health economic evaluation reporting standards 2022 (cheers 2022) statement: Updated reporting guidance for health economic evaluations // Value Health. 2022. Vol. 25, N 1. P. 3–9. doi: 10.1016/j.jval.2021.11.1351
- Морозов С.П., Владзимирский А.В., Кляшторный В.Г., и др. Клинические испытания программного обеспечения на основе интеллектуальных технологий (лучевая диагностика). Препринт № ЦДТ-2019-1. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 23. Москва: ГБУЗ НПКЦ ДиТ ДЗМ, 2019. 33 с.
- Гомболевский В.А., Блохин И.А., Лайпан А.Ш., и др. Методические рекомендации по скринингу рака легкого. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 56. Москва: ГБУЗ НПКЦ ДиТ ДЗМ, 2020. 57 с.
- Морозов С.П., Проценко Д.Н., Сметанина С.В., и др. Лучевая диагностика коронавирусной болезни (COVID-19): организация, методология, интерпретация результатов: методические рекомендации. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 65. Москва: ГБУЗ НПКЦ ДиТ ДЗМ, 2020. 78 с.
- Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Министерство здравоохранения Российской Федерации. Версия 17 (14.12.2022). Режим доступа: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/061/254/original/%D0%92%D0%9C%D0%A0_COVID-19_V17.pdf?1671088207. Дата обращения: 17.03.2023.
- Lynch D.A., Austin J.H., Hogg J.C., et al. CT-definable subtypes of chronic obstructive pulmonary disease: A statement of the Fleischner Society // Radiology. 2015. Vol. 277, N 1. P. 192–205. doi: 10.1148/radiol.2015141579
- Hersh C.P., Washko G.R., Estépar R.S., et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD // Respir Res. 2013. Vol. 146, N 1. P. 42. doi: 10.1186/1465-9921-14-42
- Walker C., Chung J.H. Muller’s imaging of the chest e-book: Expert radiology series. Elsevier Health Sciences, 2018. 1104 р.
- Erbel R., Aboyans V., Boileau C., et al.; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) // Eur Heart J. 2014. Vol. 35, N 41. P. 2873–2926. doi: 10.1093/eurheartj/ehu281
- Wanhainen A., Verzini F., Van Herzeele I., et al. Clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysmsб // Eur J Vascul Endovascul Sur. 2019. Vol. 57, N 1. P. 8–93. doi: 10.1016/j.ejvs.2018.09.020
- Jurgens P.T., Carr J.J., Terry J.G., et al. Association of abdominal aorta calcium and coronary artery calcium with incident cardiovascular and coronary heart disease events in black and white middle-aged people: The coronary artery risk development in young adults study // J Am Heart Assoc. 2021. Vol. 10, N 24. P. 23–37. doi: 10.1161/JAHA.121.023037
- Galiè N., Humbert M., Vachiery J.L., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT) // Eur Respir J. 2015. Vol. 46, N 4. P. 903–975. doi: 10.1183/13993003.01032-2015
- Agatston A.S., Janowitz W.R., Hildner F.J., et al. Quantification of coronary artery calcium using ultrafast computed tomography // J Am Coll Cardiol. 1990. Vol. 15, N 4. P. 827–832. doi: 10.1016/0735-1097(90)90282-t
- Журавлев К.Н. КТ-коронарография. Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 65. Москва: ГБУЗ НПК ЦДиТ ДЗМ, 2020. 35 с.
- Mayo-Smith W.W., Song J.H., Boland G.L., et al. Management of incidental adrenal masses: A white paper of the ACR incidental findings committee // J Am Coll Radiol. 2017. Vol. 14, N 8. P. 1038–1044. doi: 10.1016/j.jacr.2017.05.001
- Белая Ж.Е., Белова К.Ю., Бирюкова Е.В., и др. Федеральные клинические рекомендации по диагностике, лечению и профилактике остеопороза // Остеопороз и остеопатии. 2021. Т. 24, № 2. С. 4–47. doi: 10.14341/osteo12930
- Genant H.K., Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research // Osteoporosis Int. 2003. Vol. 14, N 3. P. 43–55. doi: 10.1007/S00198-002-1348-1
- Патологические переломы, осложняющие остеопороз. Клинические рекомендации. Москва, 2018. 90 с. Режим доступа: https://neirodoc.ru/wp-content/uploads/2019/10/patologicheskie-perelomy-oslozhnyayuschie-osteoporoz-2018.pdf. Дата обращения: 17.03.2023.
- Колбин А.С., Гомон Ю.М., Балыкина Ю.Е., и др. Социально-экономическое и глобальное бремя COVID-19 // Качественная клиническая практика. 2021. № 1. С. 24–34. doi: 10.37489/2588-0519-2021-1-24-34
- Pickhardt P.J., Correale L., Hassan C. AI-based opportunistic CT screening of incidental cardiovascular disease, osteoporosis, and sarcopenia: Cost-effectiveness analysis // Abdom Radiol (NY). 2023. N 3. P. 1181–1198. doi: 10.1007/s00261-023-03800-9
- Behr C.M., Koffijberg H., Degeling K., et al. Can we increase efficiency of CT lung cancer screening by combining with CVD and COPD screening? Results of an early economic evaluation // Eur Radiol. 2022. Vol. 32, N 5. P. 3067–3075. doi: 10.1007/s00330-021-08422-7
- Chintanapakdee W., Mendoza D.P., Zhang E.W., et al. Detection of extrapulmonary malignancy during lung cancer screening: 5-year analysis at a tertiary hospital // J Am Coll Radiol. 2020. Vol. 17, N 12. P. 1609–1620. doi: 10.1016/j.jacr.2020.09.032
- Girling A., Lilford R., Cole A., et al. Headroom approach to device development: Current and future directions // Int J Technol Assess Health Care. 2015. Vol. 31, N 5. P. 331–338. doi: 10.1017/S0266462315000501
- Ijzerman M.J., Steuten L.M. Early assessment of medical technologies to inform product development and market access: A review of methods and applications // Appl Health Econ Health Policy. 2011. Vol. 9, N 5. P. 331–347. doi: 10.2165/11593380-000000000-00000
- Izerman M.J., Koffijberg H., Fenwick E., et al. Emerging use of early health technology assessment in medical product development: A scoping review of the literature // Pharmacoeconomics. 2017. Vol. 35, N 7. P. 727–740. doi: 10.1007/s40273-017-0509-1
- Sahiner B., Pezeshk A., Hadjiiski L.M., et al. Deep learning in medical imaging and radiation therapy // Med Phys. 2019. Vol. 46, N 1. P. 1–36. doi: 10.1002/mp.13264
- Allen B.J., Seltzer S.E., Langlotz C.P., et al. A road map for translational research on artificial intelligence in medical imaging: From the 2018 national institutes of health/RSNA/ACR/The academy workshop // J Am Coll Radiol. 2019. Vol. 16, N 9. P. 1179–1189. doi: 10.1016/j.jacr.2019.04.014
- Xia C., Rook M., Pelgrim G.J., et al. Early imaging biomarkers of lung cancer, COPD and coronary artery disease in the general population: Rationale and design of the ImaLife (Imaging in Lifelines) Study // Eur J Epidemiol. 2020. Vol. 35, N 1. P. 75–86. doi: 10.1007/s10654-019-00519-0
- Ziegelmayer S., Graf M., Makowski M., et al. Cost-effectiveness of artificial intelligence support in computed tomography-based lung cancer screening // Cancers (Basel). 2022. Vol. 14, N 7. P. 1729. doi: 10.3390/cancers14071729
- McDonald R.J., Schwartz K.M., Eckel L.J., et al. The effects of changes in utilization and technological advancements of cross-sectional imaging on radiologist workload // Acad Radiol. 2015. Vol. 22, N 9. P. 1191–1198. doi: 10.1016/j.acra.2015.05.007
- Sokolovskaya E., Shinde T., Ruchman R.B., et al. The effect of faster reporting speed for imaging studies on the number of misses and interpretation errors: A pilot study // J Am Coll Radiol. 2015. Vol. 12, N 7. P. 683–688. doi: 10.1016/j.jacr.2015.03.040
- Geijer H., Geijer M. Added value of double reading in diagnostic radiology, a systematic review // Insights Imaging. 2018. Vol. 9, N 3. P. 287–301. doi: 10.1007/s13244-018-0599-0
- Lauritzen P.M., Hurlen P., Sandbæk G., et al. Double reading rates and quality assurance practices in Norwegian hospital radiology departments: Two parallel national surveys // Acta Radiol. 2015. Vol. 56, N 1. P. 78–86. doi: 10.1177/0284185113519988
- Морозов С.П., Чернина В.Ю., Андрейченко А.Е., и др. Как искусственный интеллект влияет на оценку поражения лёгких при COVID-19 по данным КТ грудной клетки? // Digital Diagnostics. 2021. Т. 2, № 1. C. 27–38. doi: 10.17816/DD60040
Дополнительные файлы