К вопросу об этических аспектах внедрения систем искусственного интеллекта в здравоохранении
- Авторы: Шарова Д.Е.1, Зинченко В.В.1, Ахмад Е.С.1, Мокиенко О.А.1, Владзимирский А.В.1, Морозов С.П.1
-
Учреждения:
- Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
- Выпуск: Том 2, № 3 (2021)
- Страницы: 356-368
- Раздел: Обзоры
- Статья получена: 06.08.2021
- Статья одобрена: 08.09.2021
- Статья опубликована: 15.10.2021
- URL: https://jdigitaldiagnostics.com/DD/article/view/77446
- DOI: https://doi.org/10.17816/DD77446
- ID: 77446
Цитировать
Аннотация
Рассматриваются этические вопросы применения в ходе жизненного цикла систем искусственного интеллекта; представлена актуальная информация о мировых и отечественных тенденциях в этой сфере. Описан международный и национальный опыт в области этических вопросов применения систем искусственного интеллекта в здравоохранении; разобраны международные и национальные стратегии развития искусственного интеллекта в здравоохранении, где особое внимание уделяется национальному развитию, и выявлены основные тенденции, сходства и различия между стратегиями. Описаны также этические составляющие процесса клинических испытаний систем искусственного интеллекта в России, в рамках которых оцениваются их безопасность и эффективность. В рамках передового отечественного опыта по техническому регулированию систем искусственного интеллекта, аналогов которому нет в мире, представлены работы по унификации и стандартизации требований, используемые при разработке, тестировании и эксплуатации систем искусственного интеллекта в здравоохранении и уникальный опыт России в части сертификационных требований к медицинским изделиям, использующим технологии искусственного интеллекта.
Особо подчёркнута важность построения успешной системы здравоохранения в области технологий искусственного интеллекта, которая способствует укреплению доверия и соблюдению этических норм.
Ключевые слова
Полный текст
ВВЕДЕНИЕ
Искусственный интеллект представляет собой комплекс технологических решений, позволяющий имитировать когнитивные функции человека (включая самообучение, поиск решений без заранее заданного алгоритма и достижение инсайта) и получать при выполнении конкретных практически значимых задач обработки данных результаты, сопоставимые как минимум с результатами интеллектуальной деятельности человека, согласно ГОСТ Р 59277-2020. Таким образом, системы на основе технологий искусственного интеллекта (СИИ) имеют определённые особенности, включая способность к «эволюции», работу с «новыми знаниями», что накладывает ответственность на общество по разработке новых этических норм в вопросах применения подобных систем (помощь врачу в диагностике заболеваний и принятии решений, в целом помощь в медицине). СИИ должны быть не только эффективными за счёт своих широких возможностей, но и безопасными, за что в свою очередь отвечают нормативное, в том числе этические нормы (создание этического кодекса, национальные и международные стандарты по этике в области технологий искусственного интеллекта в медицине) и техническое регулирование, которое активно обновляется и формируется. Несмотря на подобие чёрному ящику, СИИ, благодаря формирующимся отлаженным нормам и правилам работы с ними, не должны вызывать недоверия, ведь преимуществ использования технологий искусственного интеллекта гораздо больше, включая уменьшение кадрового дефицита и получение независимого второго мнения.
Развитие СИИ поднимает этические вопросы нового типа, касающиеся влияния на процессы принятия решений, взаимодействие в медицине, систему здравоохранения в целом [1]. В течение жизненного цикла СИИ важно соблюдать права человека, а для этого необходимо систематически обсуждать этические аспекты, а также устанавливать определённые этические нормы применения СИИ в глобальном масштабе [2].
Этическая часть характерна для всех этапов жизненного цикла СИИ, представляющего собой совокупность процессов ― от разработки (научные, в том числе клинические исследования, проектирование, создание), эксплуатации (вывод на рынок, финансирование, техническое обслуживание, мониторинг и оценка эффективности, контроль работоспособности) и до вывода из эксплуатации. Особенно актуально рассмотрение этических вопросов в процессе жизненного цикла при взаимодействии объектов с СИИ, при этом под задействованными с СИИ объектами понимаются любые стороны, вовлечённые в этапы жизненного цикла СИИ, ― юридические и физические лица (исследователи ― научные сотрудники, врачи; специалисты по обработке данных; инженеры; специалисты в сфере информационных технологий; коммерческие и государственные компании, университеты, научно-исследовательские центры и т.д.
Отдельно стоит отметить этический вопрос применения искусственного интеллекта в системах мирового [3] и отечественного здравоохранения [4]. СИИ, которые являются медицинским изделием, становится всё больше, поэтому необходимо исключить или максимально снизить возможность возникновения ошибок, связанных с применением медицинских и персональных данных пациентов, используемых для разработки и тестирования в области технологий искусственного интеллекта. Для выполнения указанных задач необходим доступ к достаточному объёму медицинских данных пациентов, которые соответствуют высоким стандартам качества (верифицированы должным образом) [5] и могут использоваться не только в прогнозировании заболевания, диагностике и оптимизации лечения [6], но и для внешних и внутренних тестирований алгоритмов [7]. Таким образом, для решения этических проблем, связанных с разработкой и внедрением СИИ в медицину, необходим комплекс решений, включающий регулирование со стороны государства, работу высококвалифицированных экспертов (инженеры, врачи, ИТ-специалисты и др.) и повышение уровня осведомлённости пациентов.
Цель данного обзора ― представление имеющихся на данный момент документов, регламентирующих этические аспекты и нормы применения искусственного интеллекта в мире, в частности в отрасли здравоохранения, а также исследование нормативных правовых и технических регулирующих документов для СИИ в мире с представлением российского опыта первых национальных стандартов, которые будут контролировать разработку и применение технологий искусственного интеллекта в здравоохранении.
МЕЖДУНАРОДНЫЙ И НАЦИОНАЛЬНЫЙ ОПЫТ В ОБЛАСТИ ЭТИЧЕСКИХ ВОПРОСОВ ПРИМЕНЕНИЯ СИСТЕМ НА ОСНОВЕ ТЕХНОЛОГИЙ ИСКУССТВЕННОГО ИНТЕЛЛЕКТА В ЗДРАВООХРАНЕНИИ. ОБЩИЕ ПОЛОЖЕНИЯ
Широко распространённое использование данных пациентов для обучения СИИ вызывает очевидные опасения по поводу обеспечения конфиденциальности данных. Сохранение баланса с целью улучшения медицинского обслуживания между вторичным использованием данных других пациентов и конфиденциальностью персональной информации вызывает ряд проблем. Например, на уровне отдельных пациентов проблемой является понимание, в какой степени их персональные данные подвергаются вторичному использованию и какие элементы их задействованы; кто может получить доступ к их данным; насколько анонимность данных эффективна и полна; могут ли эти данные потенциально использоваться для нанесения им вреда; можно ли изменить их данные; используются ли их данные для финансовой выгоды других лиц и повлияет ли изменение политики конфиденциальности данных в ближайшем или отдалённом будущем на получаемую ими помощь [6]. На уровне медицинских учреждений, между которыми происходит обмен медицинскими данными, встаёт вопрос «осознания ответственности» за владение персональными данными пациентов, и может возникнуть препятствующая такому обмену проблема.
Вместе с тем в случае постановки врачом неверного диагноза при использовании СИИ не ясно, в какой степени каждая вовлечённая сторона (врач, учреждение, система здравоохранения или производитель СИИ) несёт этическую ответственность. Согласно существующим нормативным правовым актам [ст. 98 Федерального закона от 21.11.2011 № 323-ФЗ (ред. от 02.07.2021) «Об основах охраны здоровья граждан в Российской Федерации»], ответственность за нарушение различных правовых норм в сфере охраны здоровья на данный момент времени лежит на медицинской организации и враче.
На государственном уровне ответственность за медицинские ошибки становится ещё более неоднозначной. СИИ могут не отвечать всем установленным к ним требованиям, существуют риски при применении диагностических медицинских устройств, в том числе СИИ. Управление по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA) заявляет, что диагностические медицинские устройства могут нести определённые риски по пяти причинам [8]: увеличение ложноположительных результатов, приводящих к ненужным дополнительным процедурам; увеличение ложноотрицательных результатов (невозможность диагностировать заболевание); применение к неподходящим группам населения; неправомерное использование людьми; неисправность из-за неправильного вывода продукта (СИИ) на рынок. Таким образом, чтобы минимизировать риски, необходима прозрачная и налаженная система регулирования со стороны государства, что позволит частично снять (контролировать) этические вопросы применения СИИ на государственном уровне.
По определению, система регулирования ― это ряд правил. В отношении продуктов, производимых промышленностью, таких как лекарственные средства, вакцины и устройства медицинского назначения, в том числе СИИ, роль правил состоит в том, чтобы ограничивать риск нанесения продуктом вреда (небезопасный продукт), риск несоответствия цели, для которой был предназначен продукт (неэффективный продукт), или риск несоответствия стандартам качества на основании глобального доклада Всемирной организации здравоохранения «Ethics and governance of artificial intelligence for health» [9]. Национальное законодательство отвечает за разработку таких правил и создание условий, обеспечивающих выполнение этих законов. Среди тех, кто обязан следовать этим правилам, ― производители и пользователи СИИ (медицинские организации, врачи и другой медицинский персонал). Например, производителю СИИ рекомендуется использовать принятую классификацию патологий согласно утверждённым тезаурусам и Международной классификации болезней либо наименование феноменов в соответствии с рекомендациями профильной ассоциации специалистов.
ОБЩИЕ ПОДХОДЫ К РЕШЕНИЮ ПОСТАВЛЕННЫХ ЭТИЧЕСКИХ ПРОБЛЕМ
Эксперты Всемирной организации здравоохранения (ВОЗ) сформулировали принципы решения поставленных этических проблем [9], которые применимы к искусственному интеллекту в здравоохранении.
1. Защита автономности
С развитием искусственного интеллекта врачи начали высказывать опасения, что данные технологии смогут их заменить, однако предложенный принцип защиты автономности подразумевает, что СИИ должны разрабатываться для помощи в принятии решения и быть полностью контролируемыми при эксплуатации медицинским персоналом. Кроме того, данный принцип подразумевает под собой уважение к защите данных и их конфиденциальности со стороны производителей СИИ, создателей наборов данных, правительством стран и др., что должно быть закреплено в нормативных правовых актах. В России на территории Москвы введён экспериментальный режим обработки персональных данных (Федеральный закон от 27 июля 2006 г. № 152-ФЗ «О персональных данных»), что позволило внести изменения в информированные согласия пациентов и обеспечить реализацию данного принципа. Кроме того, в 2020 году были инициированы изменения в нормативных правовых документах, связанных с медицинскими изделиями. Данные поправки обозначили роль СИИ как помощь в принятии врачебного решения, что позволило снять вопрос применения искусственного интеллекта без вмешательства врача.
2. Обеспечение безопасности и благополучия пациента
СИИ должны быть разработаны таким образом, чтобы не причинять физического и морального вреда пациенту. Они должны соответствовать утверждённым требованиям по безопасности, эффективности и качеству на протяжении всего периода эксплуатации. Предлагается, что для этих целей будут внедрены механизмы контроля качества и мониторинг, чтобы СИИ функционировали так, как это было предназначено. Были утверждены рекомендации Международного форума регуляторов медицинских изделий по выполнению клинической оценки программного обеспечения, являющегося медицинским изделием, которые адаптируются в России [10]. Ведутся также разработки стандартов протоколов и отчётов по результатам клинических исследований. Например, рандомизированные контролируемые испытания считаются золотым стандартом экспериментального дизайна для предоставления доказательств безопасности и эффективности СИИ. В связи с этим разработано расширение CONSORT-AI (Consolidated Standards of Reporting Trials — Artificial Intelligence) к стандартам CONSORT 2010 и SPIRIT-AI (Стандартные элементы протокола: рекомендации для интервенционных испытаний — Искусственный интеллект) [11].
3. Обеспечение прозрачности, объяснимости и понятности СИИ
Понятность СИИ включает обеспечение прозрачности и объяснимости. Производители СИИ должны демонстрировать прозрачность путём публикации и представления профессиональному сообществу данных о том, каким образом была разработана модель искусственного интеллекта, каков её сценарий применения, какие были параметры наборов данных, которые применяли для разработки и валидации СИИ, какие клинические исследования выполнены, чтобы показать эффективность СИИ [10].
Алгоритмы искусственного интеллекта, как и принцип обработки данных и принятия решения, должны быть объяснимы максимально возможно, чтобы информировать субъектов персональных данных, пациентов.
Указанный принцип понятности СИИ также связан с принципом обеспечения безопасности, т.к. требует раскрытия информации о проведённых тестированиях, их полноте и качестве.
4. Повышение ответственности и подотчётности
Инженеры и производители СИИ совместно с медицинскими специалистами должны разработать и продумать сценарии применения данной технологии, в том числе какие результаты эффективности и производительности необходимо получить. При этом ответственностью заинтересованных сторон является применение СИИ по назначению и в рамках заявленных условий. Все случаи некорректных срабатываний СИИ должны быть задокументированы, и в дальнейшем проведён их мониторинг.
5. Обеспечение равенства
Технологии искусственного интеллекта должны быть доступны для всех слоёв общества, независимо от пола, расы, возраста и достатка, а также занимаемой должности и т.д. Производители СИИ должны убедиться и представить информацию, что наборы данных для обучения и тестирования искусственного интеллекта не содержат систематических ошибок (смещений) выборки и, следовательно, являются точными, полными и разнообразными, что даёт равные параметры эффективности СИИ в рамках всей популяции.
6. Обеспечение обратной связи и стабильности искусственного интеллекта
Обеспечение обратной связи требует от производителя и пользователя СИИ непрерывной сбора и анализа информации о применении СИИ с целью контролировать соответствие её работы с заявленными требованиями. Необходимо обеспечивать параметры ресурсов и внешних систем для функционирования СИИ, а также её интеграцию, например, в медицинские информационные системы. Обратная связь актуальна для оценки места конкретной СИИ в системе здравоохранения, пожеланий врачей, выполнения и эффективности предполагаемых сценариев применения искусственного интеллекта.
Представленные принципы решения этических проблем являются общими и должны быть адаптированы к национальным особенностям. Многие мировые державы представили свои национальные стратегии, в которых закрепили основные принципы развития искусственного интеллекта, включающие и указанные ранее.
МЕЖДУНАРОДНЫЕ СТРАТЕГИИ РАЗВИТИЯ ИСКУССТВЕННОГО ИНТЕЛЛЕКТА В ЗДРАВООХРАНЕНИИ
В целях исследования международных стратегий использования и развития искусственного интеллекта в здравоохранении были рассмотрены документы мировых ведущих держав.
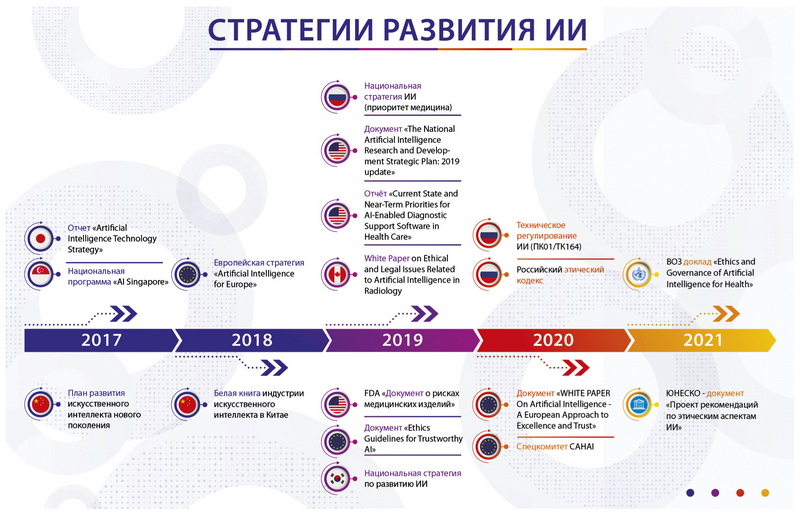
В настоящее время большинство мировых держав, в их числе США, Китай, Япония, Южная Корея, страны Европы и Россия, разработали и опубликовали национальные стратегии или программные документы (рисунок), определяющие цели, задачи и планы развития СИИ, в каждом из которых особое внимание уделяется этической составляющей в здравоохранении [12, 13].
Япония ― одна из первых стран, где была разработана национальная стратегия искусственного интеллекта. В 2017 году был выпущен отчёт «Artificial Intelligence Technology Strategy» [14], согласно которому здравоохранение является одной из основных отраслей для развития. В 2017 году Сингапур запустил национальную программу «AI Singapore» [15], фокусом которой является национальная система здравоохранения. Южная Корея в 2019 году опубликовала свою национальную стратегию по развитию искусственного интеллекта [16], в которой также приоритетное место выделено системе здравоохранения.
Европейская комиссия в 2018 году приняла стратегию искусственного интеллекта «Artificial Intelligence for Europe» [17], в которой представлена европейская инициатива по искусственному интеллекту, направленная на обеспечение соответствующих этических и правовых подходов путём создания Европейского альянса искусственного интеллекта и разработки руководящих принципов этики искусственного интеллекта.
Рис. Временная шкала разработки и утверждения стратегий развития искусственного интеллекта в мире.
В 2019 году Соединённые Штаты Америки опубликовали документ «The National artificial intelligence research and development strategic plan: 2019 update» [12], в котором определены 8 стратегических приоритетов: долгосрочные инвестиции; эффективные методы взаимодействия человека и искусственного интеллекта; регулирование правовых и этических последствий применения искусственного интеллекта; безопасность и защита искусственного интеллекта; общедоступные наборы данных; стандартизация и тестирование искусственного интеллекта; подготовка кадров и частно-государственное партнёрство. В том же году был опубликован отчёт «Current State and Near-Term Priorities for AI-Enabled Diagnostic Support Software in Health Care» [18], в котором экспертами обоснована необходимость изменения государственной политики и нормативного регулирования для обеспечения более безопасного и эффективного применения искусственного интеллекта в здравоохранении. В этом документе представлен обзор существующей нормативно-правовой базы, регулирующей разработку и применение систем поддержки принятия врачебных решений и СИИ для поддержки диагностики. Эксперты сделали выводы о необходимости изменения нормативного регулирования СИИ для здравоохранения на основе следующих постулатов:
- Предоставление доказательств, что СИИ улучшает результаты лечения пациентов, повышает качество и снижает стоимость этого лечения, предоставляет врачам соответствующую информацию, которая является «полезной и заслуживающей доверия».
- Оценка потенциального риска использования продуктов искусственного интеллекта в клинических условиях. «Степень, в которой программный продукт поставляется, с информацией, объясняющей, как он работает, а также описывает те популяции, которые были использованы для машинного обучения, будет иметь значительное влияние на оценку регуляторами и клиницистами риска применения таких продуктов для пациентов, ― отмечают исследователи. ― Возможно, потребуется пересмотреть маркировку продукции и обсудить риски и преимущества непрерывного обучения по сравнению с закрытыми моделями».
В 2019 году Канада опубликовала «Белую книгу по этическим и юридическим аспектам, связанным с искусственным интеллектом в радиологии» [6]. Официальный документ Канадской ассоциации радиологов представляет собой основу для изучения юридических и этических вопросов, связанных с искусственным интеллектом в медицинской визуализации. Особое внимание уделено вопросам конфиденциальности данных пациентов, СИИ (уровни автономии, ответственности и юридические аспекты); канадскому практическому опыту в здравоохранении (лучшие практики и действующая правовая база) и прогнозируемым возможностям, которые сможет обеспечить искусственный интеллект для системы здравоохранения.
Опубликованное в апреле 2019 года группой экспертов Европейской комиссии «Руководство по этике для надёжности искусственного интеллекта» [19] содержит ряд ключевых требований, соответствие которым возводит СИИ в степень технологий, заслуживающих доверия: человеческое участие и надзор; техническая надёжность и безопасность; конфиденциальность и управление данными; прозрачность; разнообразие; недискриминация и справедливость; экологическое и социальное благополучие; ответственность.
Осенью 2020 года Европейский парламент предложил сформировать новую правовую основу с изложением этических принципов и правовых обязательств при разработке, развёртывании и использовании искусственного интеллекта и робототехники ― «Белую книгу по регулированию искусственного интеллекта» [3].
Совет Европы (Спецкомитет по искусственному интеллекту CAHAI) [20], в состав которого входят представители России, активно разрабатывает подходы к европейскому регулированию.
ЮНЕСКО разработал проект рекомендаций по этике искусственного интеллекта [21]: документ планируется к принятию странами-членами ЮНЕСКО в конце 2021 года. Европа становится глобальным игроком в этике искусственного интеллекта. Европейским парламентом активно разрабатываются документы по этике искусственного интеллекта, в том числе в здравоохранении [20].
Стоить также отметить, что в США используется более свободный рыночный подход к СИИ, чем в Европе, и что рынок медицинских изделий (программного обеспечения на основе ИИ) США ― один из самых крупных в мире. Согласно прогнозу, искусственный интеллект может внести в мировую экономику до 13,33 трлн евро в 2030 году, и регионами, которые, по оценкам, больше всего выиграют от искусственного интеллекта, скорее всего, будут Китай и Северная Америка, а затем Южная Европа [22].
Стратегия развития сферы искусственного интеллекта в Китае соотносится со стратегией научно-технологического развития РФ, приоритетные направления которой заключаются в переходе к «цифровым, интеллектуальным производственным технологиям, роботизированным системам, новым материалам и способам конструирования, создания систем обработки больших объёмов данных, машинного обучения и искусственного интеллекта» [23]. Госсовет КНР в 2017 г. опубликовал «План развития искусственного интеллекта нового поколения», в котором обозначены поэтапные цели развития искусственного интеллекта, а в 2018 году была разработана «Белая книга индустрии искусственного интеллекта в Китае» [24]. В обоих документах в числе приоритетных направлений указаны здравоохранение и этические аспекты.
Отдельные работы поднимают вопрос этичной разработки и внедрения СИИ в медицинских сообществах. Например, совместное заявление Европейского и Североамериканского сообществ поднимает основные принципы этики, аналогичные предложенным ВОЗ [25]; сообщества в радиологии заявляют о намерении разработки единого этического кодекса с целью обеспечить надёжную этическую основу по мере быстрого развития технологий [26].
В России в настоящий момент приоритетным является развитие и внедрение искусственного интеллекта в здравоохранение, что должно способствовать достижению стратегических целей и задач, предусмотренных национальным проектом «Здравоохранение», включая сокращение заболеваемости и смертности, рост ожидаемой продолжительности жизни и т.д. Подробнее рассмотрим национальную стратегию в следующем разделе.
НАЦИОНАЛЬНАЯ СТРАТЕГИЯ РАЗВИТИЯ ИСКУССТВЕННОГО ИНТЕЛЛЕКТА В ЗДРАВООХРАНЕНИИ
В России национальные процессы в сфере развития искусственного интеллекта, в том числе в здравоохранении, построены на принципах открытости, большой вовлечённости научных сотрудников, производителей СИИ и государственных структур. В 2020 году по поручению президента РФ была начата работа над российским этическим кодексом в сфере искусственного интеллекта [27].
Россия придаёт сегодня большое значение международному сотрудничеству по развитию СИИ [28], при этом важно правильно распорядиться возможностями искусственного интеллекта, создать необходимые основы для безопасного и этичного развития СИИ, стандартизировать подходы к её разработке, чтобы она несла в себе созидательную силу и способствовала развитию потенциала человека и его способностей.
Россия, являясь активным участником процесса по развитию искусственного интеллекта, следует всем мировым тенденциям. Российское правительство разработало и приняло комплексный пакет программных документов, которые позволили приступить к реализации национальной стратегии [29]. В октябре 2019 года Президент РФ В.В. Путин подписал Указ № 490 «О развитии искусственного интеллекта в Российской Федерации», который вводит «Национальную стратегию развития искусственного интеллекта на период до 2030 года» и утверждает планы инициации федерального проекта «Искусственный интеллект» (введён в 2020 году в рамках национального проекта «Цифровая экономика Российской Федерации») [30]. В рамках федерального проекта предусмотрено 6 основных блоков: поддержка научных исследований; создание комплексной системы правового регулирования; разработка и развитие программного обеспечения; повышение доступности и качества данных; повышение доступности аппаратного обеспечения; подготовка квалифицированных кадров и повышение уровня информированности населения о технологиях на основе искусственного интеллекта.
Особенностью национальной стратегии развития искусственного интеллекта в России, отличающей её от большинства национальных стратегий других стран, является приоритет в развитии СИИ для здравоохранения, в том числе подчёркнута необходимость перехода от общих принципов к более практическому уровню. Одним из таких путей практического развития искусственного интеллекта в России является нормативное правовое и техническое (стандартизация) регулирование. В России программное обеспечение, созданное с применением технологий искусственного интеллекта и предназначенное производителем для использования при оказании медицинской помощи, относится к программным медицинским изделиям (программное обеспечение, являющееся медицинским изделием).
Для развития технического регулирования в июле 2019 года создан технический комитет по стандартизации «Искусственный интеллект» (ТК164), который занимается вопросами искусственного интеллекта и повышения эффективности работ по стандартизации в данной области [31]. В рамках ТК164 создан подкомитет ПК01 «Искусственный интеллект в здравоохранении», разрабатывающий национальные и международные стандарты, которые распространяются на требования к разработке, проведению испытаний, а также применению и эксплуатации медицинского программного обеспечения, работающего на основе искусственного интеллекта. ПК01 создан в целях координации работ по унификации и стандартизации требований, используемых при разработке, тестировании и эксплуатации систем искусственного интеллекта в здравоохранении, а также для установки сертификационных требований к медицинским изделиям, использующим технологии искусственного интеллекта [32].
В целях регулирования процесса клинических испытаний СИИ (как части клинической оценки в ходе независимых тестирований разрабатываемых СИИ), в рамках которых оценивается их безопасность и эффективность, а также для регистрации СИИ как медицинских изделий в настоящее время российскими экспертами разработан и подготовлен к утверждению соответствующий стандарт. Этическую экспертизу рекомендуется проводить перед проведением клинических испытаний незарегистрированных медицинских изделий (СИИ). В рамках этической экспертизы предусмотрены вопросы, связанные с этическими составляющими в сфере применения искусственного интеллекта, включая анализ возможных неблагоприятных событий, возникающих при использовании СИИ. Этическим комитетом также должна быть рассмотрена программа клинических испытаний (в части одобрения дизайна испытаний, контроля формирования наборов данных), проведена оценка соответствия квалификации исследователей предлагаемому испытанию.
Стоит отметить, что вызов последних лет ― пандемия COVID-19 ― обострил понимание этических аспектов применения искусственного интеллекта в здравоохранении, в том числе для диагностики данного заболевания с помощью компьютерной томографии органов грудной клетки [33]. В работе показано, что технологии искусственного интеллекта могут корректировать поток пациентов по категориям выраженности рентгенологических признаков изменений в лёгких при COVID-19, что может влиять на тактику ведения таких пациентов. Это объясняется тем, что системы искусственного интеллекта проводят автоматическую обработку изображений пациента и, соответственно, точнее оценивают объём изменений в лёгких, в то время как врачи без применения искусственного интеллекта выполняют такую оценку визуально. Другим этическим аспектом применения искусственного интеллекта в период пандемии COVID-19 стал вопрос обеспечения конфиденциальности, защиты данных и их использования в целях отслеживания вируса и др. [21].
ЗАКЛЮЧЕНИЕ
В настоящее время ведётся активная международная и национальная разработка нормативов регулирования СИИ в здравоохранении, однако на текущий момент зарубежными и национальными регулирующими органами не сформированы полноценная нормативная правовая база и системы контроля, недостаточен опыт работы в области технологий искусственного интеллекта, остаются нерешёнными или спорными этические аспекты применения подобных технологий.
В качестве первоочередных мер по совершенствованию и доработке нормативных и правовых документов в России активно развивается не только юридическое, но и техническое регулирование СИИ, изучаются и прорабатываются вопросы оценки, измерения и стандартизации технологий искусственного интеллекта. Наиболее активно ведётся работа в области национальной отраслевой стандартизации здравоохранения. Унификация лучших практик позволит структурировать разработку, оценку, регулирование и контроль безопасности в области применения и разработки технологий искусственного интеллекта и повысит к ним доверие общества.
Необходимо создать систему, основанную на выполнении этических норм для достижения желаемой общественной цели, чтобы СИИ приносили пользу обществу. В связи с этим необходимо не только переосмыслить существующую нормативную правовую базу, но и обновить её с учётом новых технологических достижений. Согласно нормативным правовым актам, а также в рамках разработки отраслевых стандартов в клинической медицине, необходимо разрабатывать и оценивать СИИ в соответствии с требованиями этических норм, особенно для врачей и других представителей медицинского сообщества. СИИ должна быть «прозрачной» в понимании для медицинских работников и защищать интересы врачей и пациентов, особенно в области этического регулирования ― только в этом случае удастся наладить доверительные отношения между врачом, пациентом и производителем СИИ с соблюдением норм этической безопасности.
Международному сообществу и частным компаниям следует разработать механизмы контроля и проверки соблюдения этических норм, что позволит как выявлять, предупреждать и минимизировать воздействие СИИ с точки зрения соблюдения прав человека, принципов надлежащей научной практики, законности и защиты, так и оценивать эффективность таких механизмов. Государствам предлагается рассмотреть возможность использования таких форм управления, как механизм сертификации СИИ и взаимное признание стандартизации, принимая при этом во внимание особенности отрасли здравоохранения, применение СИИ и их потенциальное воздействие на жизнь людей, а также другие этические аспекты.
Безопасность и защищённость СИИ может быть повышена посредством разработки надёжных систем от несанкционированного доступа к личной информации комплексных решений, которые обеспечат более эффективное обучение моделей искусственного интеллекта на основе качественных данных.
Важно, чтобы общественные и политические дискуссии были сосредоточены на этике здравоохранения. Искусственный интеллект обладает огромным потенциалом для улучшения системы здравоохранения, но раскрыть его возможно только при условии, если прямо сейчас приступить к решению стоящих перед обществом этических проблем.
Является ли использование СИИ этически приемлемым, зависит от того, насколько технологии искусственного интеллекта будут способствовать положительному вкладу для отдельных пациентов и общества в целом, включая медицинские организации, врачей (пользователей). На всех уровнях взаимодействия важно руководствоваться относительным уровнем равенства между принципами справедливости и ожидаемыми положительными результатами.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Статья подготовлена при поддержке Департамента здравоохранения города Москвы в рамках Программы «Научное обеспечение столичного здравоохранения» на 2020–2022 гг. (№ ЕГИСУ: АААА-А21-121012290079-2).
Конфликт интересов. Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов. Концепция статьи — Шарова Д.Е, Зинченко В.В., Ахмад Е.С.; сбор и обработка материала, написание текста — Шарова Д.Е., Зинченко В.В., Ахмад Е.С.; составление списка литературы — Шарова Д.Е., Ахмад Е.С.; редактирование — Шарова Д.Е., Зинченко В.В., Ахмад Е.С., Мокиенко О.А., Владзимирский А.В., Морозов С.П. Все соавторы — утверждение окончательного варианта статьи, ответственность за целостность всех частей статьи. Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией).
Funding source. TThis article was prepared with support of Moscow Healthcare Department as a part of Program “Scientific Support of the Capital's Healthcare” for 2020–2022 (Unified State Information System for Accounting of Research, Development, and Technological Works No: АААА-А21-121012290079-2.
Competing interests. The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
Authors' contribution. Concept of the article — D. Sharova, V. Zinchenko, E. Akhmad; collection and processing of the materials, writing the paper — D. Sharova, V. Zinchenko, E. Akhmad; compilation of the reference list — D. Sharova, E. Akhmad; editing — D. Sharova, V. Zinchenko, E. Akhmad, O. Mokienko, A. Vladzimirsky, S. Morozov. The final version of the article was approved by all co-authors, who equally share a responsibility for the integrity of all parts of the article. All authors confirm their authorship in accordance with the international ICMJE criteria. All authors made a substantial contribution to the concept development, research and article preparation, read and approved the final version before the publication.
Об авторах
Дарья Евгеньевна Шарова
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Email: d.sharova@npcmr.ru
ORCID iD: 0000-0001-5792-3912
SPIN-код: 1811-7595
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1
Виктория Валерьевна Зинченко
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Email: v.Zinchenko@npcmr.ru
ORCID iD: 0000-0002-2307-725X
SPIN-код: 4188-0635
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1
Екатерина Сергеевна Ахмад
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Email: e.Akhmad@npcmr.ru
ORCID iD: 0000-0002-8235-9361
SPIN-код: 5891-4384
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1
Олеся Александровна Мокиенко
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Email: o.Mokienko@npcmr.ru
ORCID iD: 0000-0002-7826-5135
SPIN-код: 8088-9921
доктор медицинских наук
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1Антон Вячеславович Владзимирский
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Email: a.Vladzymyrskyy@npcmr.ru
ORCID iD: 0000-0002-2990-7736
SPIN-код: 3602-7120
доктор медицинских наук
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1Сергей Павлович Морозов
Научно-практический клинический центр диагностики и телемедицинских технологий Департамента здравоохранения г. Москвы
Автор, ответственный за переписку.
Email: Morozov@npcmr.ru
ORCID iD: 0000-0001-6545-6170
SPIN-код: 8542-1720
доктор медицинских наук, профессор
Россия, 109029, Москва, Средняя Калитниковская ул., д. 28, стр. 1Список литературы
- Тop 10 principles for ethical artificial intelligence [электронный ресурс]. UNI Global Union; 2017. Режим доступа: http://www.thefutureworldofwork.org/media/35420/uni_ethical_ai.pdf. Дата обращения: 11.07.2021.
- World Medical Association, Inc. WMA Declaration of Helsinki — Ethical Principles for Medical Research Involving Human Subjects [электронный ресурс]. Режим доступа: https://web.archive.org/web/20140101202246/http://www.wma.net/en/30publications/10policies/b3/. Дата обращения: 11.07.2021.
- Gerke S, Minssen T, Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare//Artificial Intelligence in Healthcare. 2020. P. 295–336. doi: 10.1016/B978-0-12-818438-7.00012-5
- Состояние отрасли в России и мире. Стратегия России//Альманах «Искусственный интеллект»: сборник аналитических материалов по отрасли искусственного интеллекта в России и мире. Центр компетенций НТИ по направлению «Искусственный интеллект» на базе МФТИ. 2019. № 1. C. 7–136.
- Павлов Н.А., Андрейченко А.Е., Владзимирский А.В., и др. Эталонные медицинские датасеты (MosMedData) для независимой внешней оценки алгоритмов на основе искусственного интеллекта в диагностике//Digital Diagnostics. 2021. Т. 2, № 1. C. 49–66.
- Jaremko J.L., Azar M., Bromwich R., et al. Canadian association of radiologists white paper on ethical and legal issues related to artificial intelligence in radiology//Can Assoc Radiol J. 2019. Vol. 70, N 2. P. 107–118. doi: 10.1016/j.carj.2019.03.001
- Морозов С.П., Владзимирский А.В., Кляшторный В.Г., и др. Клинические испытания программного обеспечения на основе интеллектуальных технологий (лучевая диагностика). Серия «Лучшие практики лучевой и инструментальной диагностики». Вып. 57. Москва, 2019. 51 с.
- FDA. Factors to consider regarding benefit-risk in medical device product availability, compliance, and enforcement decisions [электронный ресурс]. Режим доступа: https://www.fda.gov/ regulatory-information/search-fda-guidance-documents/factors-consider-regarding-benefit-risk-medical-device-product-availability-compliance-and. Дата обращения: 11.07.2021.
- WHO guidance. Ethics and governance of artificial intelligence for health [электронный ресурс]. 2020. Режим доступа: https://www.who.int/publications/i/item/9789240029200. Дата обращения: 11.07.2021.
- Морозов С.П., Зинченко В.В., Хоружая А.Н., и др. Стандартизация искусственного интеллекта в здравоохранении: Россия выходит в лидеры//Врач и информационные технологии. 2021. № 2. C. 12–19. doi: 1025881/18110193_2021_2_12
- Liu X., Cruz Rivera S., Moher D., et al.; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension//Nat Med. 2020. Vol. 26, N 9. P. 1364–1374. doi: 10.1038/s41591-020-1034-x
- Select Committee on artificial intelligence: a report//The national artificial intelligence research and development strategic plan: 2019 update [электронный ресурс]. 2019. Режим доступа: https://www.nitrd.gov/pubs/National-AI-RD-Strategy-2019.pdf. Дата обращения: 11.07.2021.
- Konrad Adenauer Stiftung. Comparison of national strategies to promote artificial intelligence [электронный ресурс]. Режим доступа: https://www.kas.de/en/single-title/-/content/vergleich-nationaler-strategien-zur-forderung-von-kunstlicher-intellige-1. Дата обращения: 11.07.2021.
- Artificial Intelligence Technology Strategy (Reportof Strategic Council for AI Technology) [электронный ресурс]. Strategic Council for AI Technology; 2017. Режим доступа: https://pdf4pro.com/view/artificial-intelligence-technology-strategy-nedo-6dc4e.html. Дата обращения: 11.07.2021.
- National Research Foundation. AI Singapore [электронный ресурс]. Режим доступа: https://www.nrf.gov.sg/programmes/artificial-intelligence-r-d-programme. Дата обращения: 11.07.2021.
- The International Trade Administration (ITA). Korea ― Artificial Intelligence [электронный ресурс]. Режим доступа: https://www.privacyshield.gov/article?id=Korea-Artificial-Intelligence. Дата обращения: 11.07.2021.
- European Commission website. Shaping Europe’s digital future. Communication artificial intelligence for Europe [электронный ресурс]. Режим доступа: https://digital-strategy.ec.europa.eu/en/library/communication-artificial-intelligence-europe. Дата обращения: 11.07.2021.
- Current state and near-term priorities for ai-enabled diagnostic support software in health care [электронный ресурс]. Duke-Margolis Center for Health Policy; 2019. Режим доступа: https://healthpolicy.duke.edu/sites/default/files/2019-11/dukemargolisaienableddxss.pdf. Дата обращения: 11.07.2021.
- European Commission website. Shaping Europe’s digital future. Ethics guidelines for trustworthy AI [электронный ресурс]. Режим доступа: https://digital-strategy.ec.europa.eu/en/library/ethics-guidelines-trustworthy-ai. Дата обращения: 11.07.2021.
- Council of Europe and Artificial Intelligence [электронный ресурс]. Режим доступа: https://www.coe.int/en/web/ artificial-intelligence. Дата обращения: 11.07.2021.
- ООН по вопросам образования, культуры и науки. SHS/BIO/AHEG-AI/2020/4 REV.2. Первый проект рекомендации об этических аспектах искусственного интеллекта [электронный ресурс]. 2020. Режим доступа: https://ifap.ru/pr/2020/n201116a.pdf. Дата обращения: 11.07.2021.
- European Commission website. USA-China-EU plans for AI: where do we stand? [электронный ресурс]. 2018. Режим доступа: https://ati.ec.europa.eu/sites/default/files/2020-07/USA-China-EU%20plans%20for%20AI%20-%20where%20do%20we%20stand%20%28v5%29.pdf. Дата обращения: 11.07.2021.
- Указ Президента Российской Федерации от 01.12.2016 № 642 «О стратегии научно-технологического развития Российской Федерации». Режим доступа: http://www.kremlin.ru/acts/bank/41449. Дата обращения: 11.07.2021.
- Global Artificial Intelligence Industry Whitepaper [электронный ресурс]. Deloitte Technology, Media and Telecommunications Industry; 2019. Режим доступа: https://www2.deloitte.com/cn/en/pages/technology-media-and-telecommunications/articles/global-ai-development-white-paper.html. Дата обращения: 11.07.2021.
- Geis J.R., Brady A.P., Wu C.C., et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement//Radiology. 2019. Vol. 293, N 2. P. 436–440. doi: 10.1148/radiol.2019191586
- Safdar N.M., Banja J.D., Meltzer C.C. Ethical considerations in artificial intelligence//Eur J Radiol. 2020. Vol. 122. Р. 108768. doi: 10.1016/j.ejrad.2019.108768
- Сайт Президента России. Перечень поручений по итогам конференции по искусственному интеллекту [электронный ресурс]. Режим доступа: http://www.kremlin.ru/acts/assignments/orders/64859. Дата обращения: 11.07.2021.
- Экспертный центр электронного государства. Актуальные задачи международного взаимодействия по развитию и регулированию искусственного интеллекта [электронный ресурс]. Режим доступа: http://d-russia.ru/aktualnye-zadachi-mezhdunarodnogo-vzaimodejstviya-po-razvitiyu-i-regulirovaniyu-iskusstvennogo-intellekta.html. Дата обращения: 11.07.2021.
- Распоряжение Правительства Российской Федерации от 19.08.2020 № 2129-р. Режим доступа: http://publication.pravo.gov.ru/Document/View/0001202008260005?index=0&rangeSize=1. Дата обращения: 11.07.2021.
- Указ Президента Российской Федерации от 10.10.2019 № 490 «О развитии искусственного интеллекта в Российской Федерации». Режим доступа: http://www.kremlin.ru/acts/bank/44731. Дата обращения: 11.07.2021.
- Министерство промышленности и торговли Российской Федерации. Федеральное агентство по техническому регулированию и метрологии. Приказ от 25 июля 2019 года № 1732 «О создании технического комитета по стандартизации "Искусственный интеллект"». Режим доступа: https://docs.cntd.ru/document/560916332. Дата обращения: 11.07.2021.
- Технический комитет по стандартизации «Искусственный интеллект» (ТК164). Приказ от 13.01.2020 № 1 «Об определении базовой организации подкомитета "Искусственный интеллект в здравоохранении"». Режим доступа: https://tele-med.ai/media/uploads/2021/03/18/01_-2-2.pdf. Дата обращения: 11.07.2021.
- Морозов С.П., Чернина В.Ю., Андрейченко А.Е., и др. Как искусственный интеллект влияет на оценку поражения легких при COVID-19 по данным КТ грудной клетки?//Digital Diagnostics. 2021. Vol. 2, № 1. P. 27–38. doi: 10.17816/DD60040.
Дополнительные файлы