Optimized biparametric magnetic resonance imaging protocol for prostate cancer detection
- Authors: Abuladze L.R.1, Semenov D.S.1, Panina O.Y.1,2,3, Vasilev Y.A.1
-
Affiliations:
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- City Clinical Oncological Hospital No. 1
- Moscow State University of Medicine and Dentistry named after A.I. Evdokimov
- Issue: Vol 3, No 3 (2022)
- Pages: 166-177
- Section: Technical Reports
- Submitted: 16.06.2022
- Accepted: 25.07.2022
- Published: 17.10.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/108484
- DOI: https://doi.org/10.17816/DD108484
- ID: 108484
Cite item
Abstract
BACKGROUND: Prostate cancer is one of the most commonly diagnosed cancers in men worldwide. PI-RADS v2.1 contains the requirements for the magnetic resonance imaging protocol, which cannot be fully implemented on a significant component of functioning scanners. Consequently, magnetic resonance imaging approaches vary in different medical organizations and often do not allow for a qualitative interpretation of images and diagnosis of the target pathology.
AIM: To develop a biparametric magnetic resonance imaging protocol optimized for the existing magnetic resonance imaging scanners for the diagnosis of prostate cancer and to allow the screening and detection of neoplasms as early as possible. Simultaneously, the protocol should fulfill the current PI-RADS v2.1 recommendations to the maximum possible extent and meet the requirements of effective workflow in the radiology department.
MATERIALS AND METHODS: Preliminary analysis of prostate magnetic resonance imaging scanning in medical organizations of the Moscow Health Care Department showed the absence of a unified approach. Using the iterative adjustment of scanning parameters, we adjusted the protocol to ensure acceptable quality with maximum available compliance with PI-RADS v2.1.
To quantify the quality of the images, we used the magnetic resonance imaging phantom recommended by the American College of Radiology.
RESULTS: The biparametric protocol was developed for Excelart Vantage 1.5 T, including T2-weighted images in three planes and diffusion-weighted images, which took less than 11 min. Moreover, the image quality parameters (intensity inhomogeneity, nonlinearity, resolution, and slice thickness) were within the acceptable ranges recommended by the magnetic resonance imaging manufacturer.
CONCLUSION: The prostate may be effectively evaluated using the proposed magnetic resonance imaging protocol. Introducing it into practice could have a significant impact on the detection of prostate cancer in men. The entire duration of the protocol provides a possibility to supplement it with any sequences, depending on the final purpose of investigation.
Full Text
BACKGROUND
Prostate cancer (PC) ranks second in the incidence of malignant neoplasms among men [1]. Magnetic resonance imaging (MRI) is one of the principal methods for diagnosing PC. For the first time, Prostate Imaging Reporting and Data System (PI-RADS) recommendations for evaluating the results of multiparametric MRI (mpMRI) were published in 2012 [2] and updated in 2015 [3]. The PI-RADS v.2 assessment system, however, was insufficiently perfect [4]. As a result, in 2019, a new version called PI-RADS v2.1 was released, which made it easier to assess and reduce the variability in the interpretation of prostate mpMRI by radiologists [5]. In recent years, biparametric MRI (bpMRI) has gained interest due to its shorter scanning time, which lowers the cost of the study, and its ability to avoid the frequently unjustified injection of a contrast agent. Dynamic contrast enhancement is not always decisive since it is used to detect hypointense lesions on T2-weighted images (T2- WI) and maps of the measured diffusion coefficient (MDC). In addition, the use of a contrast agent has a number of side effects, such as increased scanning time and cost of the study [6].
Experience has shown that PI-RADS recommendations, regardless of the aim of the study, cannot always be observed for a number of reasons, including established scanning protocols for a particular department of radiation diagnostics, technical characteristics of MRI scanners, their settings, and the personal preferences of radiologists and clinicians of individual medical organizations.
The study aimed to develop an accelerated (optimized) bpMRI protocol for diagnostics of PC in healthcare organizations of the Moscow City Healthcare Department (HO MHD) that adhered as closely as possible to the recommendations of PI-RADS v2.1. A protocol like this enables to reduce the time of the study to optimize the work of departments. The prostate scanning protocols can be standardized, subject to further evidence of diagnostic accuracy comparable with mpMRI. In the future, the introduction of artificial intelligence should be considered to utilize the developed algorithms to improve the quality of visualization and evaluate the results of MRI of the pelvic organs in male patients.
MATERIALS AND METHODS
Stage 1 involves the evaluation of the existing prostate scanning protocols in the HO MHD. The search and analysis of the results was performed in the Unified Radiological Information Service of the Automated Unified Medical Information System of Moscow (URIS AUMIS) for differences in the scanning technique and established technical parameters. Metadata were obtained and analyzed by uploading them from URIS AUMIS. The time interval over which the search was performed in the respective HOs included 2019–2021. The search query included the keywords “magnetic resonance imaging of the pelvic organs”, “magnetic resonance imaging of the pelvic organs with contrast”, “multiparametric magnetic resonance imaging of the prostate”, and “biparametric magnetic resonance imaging of the prostate”. The clinical cases were randomly selected by the authors.
Then, also randomly, three healthcare organizations were selected (HO 1, HO 2, and HO 3) with the most common MRI model (Excelart Vantage 1.5 T, Toshiba, Japan), and optimized protocols were established using a phantom simulating the prostate [7]. The quality of imaging was evaluated by a radiologist with more than 10 years of expertise and colleagues who worked directly on this device while scanning. From the standpoint of the process organization, the studies were conducted in the traditional method, with patients in the supine position and using a standard body coil. All patients underwent the necessary preparation before the study to reduce artifacts and reset.
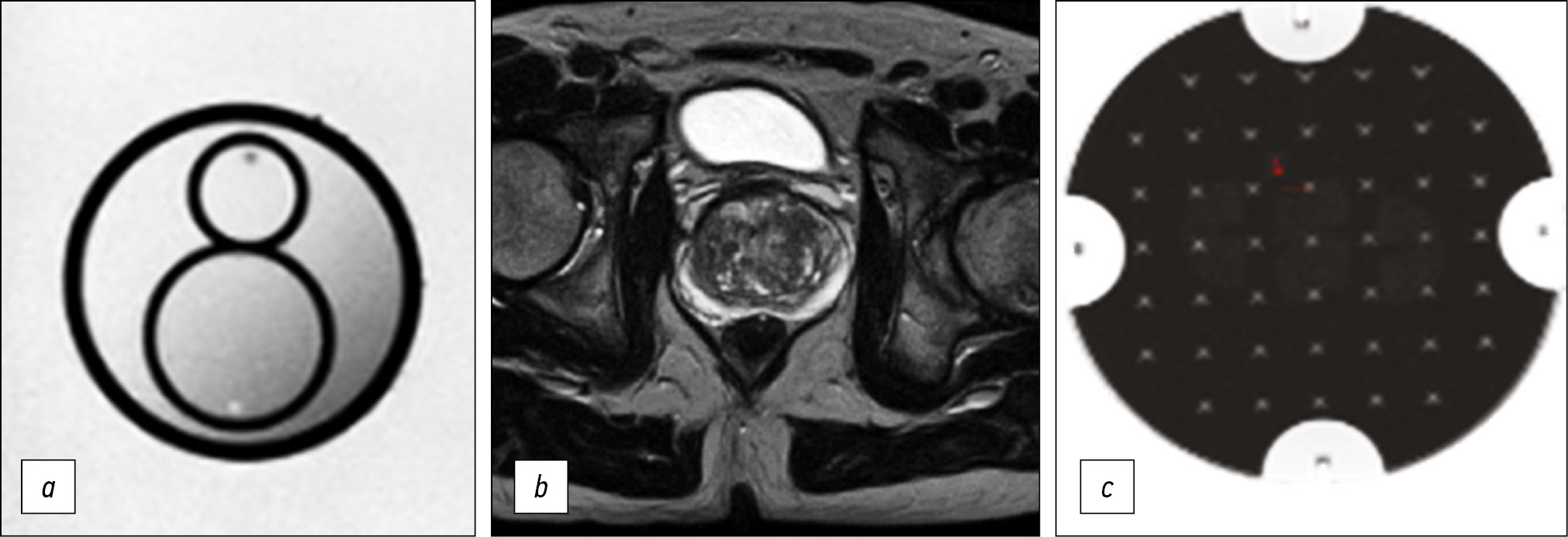
After the formation of the protocol, which adhered as closely as possible to the requirements of PI-RADS v2.1 [8] and satisfying radiologists, a quantitative assessment of the quality of imaging was performed. For this purpose, the phantom recommended by the American College of Radiology was scanned using an optimized protocol, according to the accepted technical control procedure [9] (Fig. 1). Based on the obtained axial images of the phantom, the quality parameters, namely, inhomogeneity, nonlinearity, resolution, and thickness of the selected section slice, were calculated (Table 5).
Fig. 1. Evaluation of visualization quality: a) prostate phantom; b - patient; c) phantom of the American Society of Radiology (ACR).
Table 5. Image quality control results
Parameter | Defined value | Assumed value | ||
HO 1 | HO 2 | HO 3 | ||
Heterogeneity, % | 9.44 | 5.9 | 4.8 | <10 |
Resolution, mm | 1.0 | 1.25 | 1.0 | ≤1.5 |
Nonlinearity, % | 0.1 | 0.4 | 0.2 | <1 |
Slice thickness, mm | 3.0 | 3.1 | 3.0 | 3±1 |
Note. HO, healthcare organization.
RESULTS
Evaluation of ongoing studies
When solving the problem, we discovered that different MHD HOs use different scanning technique. For example, in three HOs, the set of pulse sequences, in addition to the necessary ones, varies significantly, which affects both the completeness of diagnostic information and the total scanning time (Table 1).
Table 1. Pulse sequences used
Pulse sequence | HO 1 | HO 2 | HO 3 |
T2 ax | + (3) | + (4) | + (4) |
T2 cor | + (3) | + (3) | - |
T2 sag | + (3) | + (4) | + (5) |
T1 ax | + (3) | + (4) | - |
T1 cor | - | - | + (5) |
T1 FS cor | - | - | + (6) |
T2 FS ax | + (3) | - | + (4) |
T2 FS cor | + (3) | - | + (3) |
DWI | + (5) | + (4) | + (3) |
ADC | + (5) | + (4) | + (3) |
Total scanning time, min | 22 | 30 | 40 |
Note. “+”, presence, the layer thickness in millimeters (mm) is indicated in brackets; “-”, absence. HO, healthcare organization.
The same is true for the technical parameters of scanning. Tables 2 and 3, using T2-WI and diffusion-weighted images (DWIs) in axial view as examples, summarize the metadata that present clearly the above differences.
Table 2. Technical parameters as an example of T2-weighted images, axial view
Technical parameters | HO 1 | HO 2 | HO 3 |
TR, ms | 5851 | 6006 | 5082 |
TE, ms | 120 | 75 | 75 |
FOV, cm | 35×30 | 30×35 | 40×30 |
Matrix | 256×256 | 256×256 | 512×256 |
NAQ | 1 | 1 | 1 |
Spacing between slices, mm | 3,5 | 4,3 | 4,4 |
ETL, ms | 23 | 9 | 9 |
Note. Here and in Tables 3 and 4: TR, repetition time; TE, echo time; FOV, field of view; Matrix is a matrix; NAQ, number of data acquisitions; spacing between slices is the distance between slices; ETL, echo train length. HO, healthcare organization.
Table 3. Technical parameters as an example of diffusion-weighted images, axial view
Technical parameters | HO 1 | HO 2 | HO 3 |
TR, ms | 6772 | 9377 | 8841 |
TE, ms | 80 | 80 | 100 |
FOV, cm | 40×32 | 37×30 | 30×30 |
Matrix | 128×128 | 128×192 | 128×128 |
NAQ | 2 | 2 | 2 |
Spacing between slices, mm | 1,75 | 4,5 | 6 |
ETL, ms | 56 | 72 | 60 |
As a result, it is expected that the images obtained in different organizations will differ, as demonstrated in Fig. 2–4.
Fig. 2. Medical organization 1 (MO 1): a) T2-WI, axial projection (TR 5851, TE 120, FOV 35×30 cm, Matrix 256×256); b, c) DWI and ICD (TR 6772, TE 80, FOV 40×32 cm, Matrix 128×128).
Note. Here and in Figures 3–5: T2-WI, T2 weighted images; DWI, diffusion-weighted images; MDC, measured diffusion coefficient; TR, repetition time; TE, echo time; FOV, field of view; Matrix is a matrix.
Fig. 3. Medical organization 2 (MO 2). In the peripheral zone on the right, a hypointense zone on T2-WI and ICD map adjacent to the capsule (arrows) is defined: a) T2-WI, axial projection (TR 6006, TE 75, FOV 30×25 cm, Matrix 256×256); b, c) DWI and ICD (TR 9377, TE 80, FOV 37×30 cm, Matrix 128×192).
Fig. 4. Medical organization 3 (MO 3). In the peripheral zone on the left, a hypointense lesion on T2-WI and ICD map is defined (arrows): a) T2-WI, axial projection (TR 5082, TE 75, FOV 40×30 cm, Matrix 512×256); b, c) DWI and ICD (TR 8841, TE 100, FOV 30×30 cm, Matrix 128×128).
Thus, in all presented HO MHD, the requirements recommended by PI-RADS v2.1 for the presence of T2-WI in the axial and at least one additional (sagittal and/or coronal) views were met. In addition, it should be noted that the recommended layer thickness for T2-WI in the axial view should be no more than 3 mm, while in HO 2 and HO 3, it is 4 mm (Figures 3 and 4). The same is true for HO 1, where the DWI slice thickness is 5 mm with the recommended 4 mm or less (Figure 2). An important factor is the field of view; according to PI-RADS v2.1, field of view (FOV) values for T2-WI should be 12–20 cm, while in HO 1 and HO 3, the field of view is much larger (30 × 35 cm and 40 × 30 cm, respectively) (Figures 2 and 4). According to PI-RADS v2.1, the recommended field of view for DWI is 16–22 cm, although none of the three HOs adhere to this standard. The fact of the variability of the FOV values and the section slice thickness inevitably affects the resolution and, as a result, the ability to detect lesions.
Setting up an optimized protocol
From a technical perspective, the procedure for setting the protocol parameters did not differ from the normal operation of the applicator (e.g., when commissioning the equipment). However, in this case, we focused on the parameters recommended by PI-RADS v2.1. The parameters were preliminarily adjusted on a phantom to minimize the impact of MRI factors on the patient. Simultaneously, the values of the parameters were chosen to be as close as possible to them and iteratively adjusted to achieve a satisfactory result (from the radiologist’s standpoint) because of the impossibility of accurately following the recommendations for technical reasons. The resulting protocol is presented in Table 4.
Table 4. Optimized scan settings
Pulse sequences, layer thickness (mm) | Pre-set technical parameters | Duration (min) | |
Т2 AX (3) | TR, ms | 6400 | 2:25 |
TE, ms | 126 | ||
FOV, cm | 20×20 | ||
Matrix | 512×512 | ||
NAQ | 1 | ||
Spacing, mm | 3,3 | ||
ETL, ms | 13 | ||
Т2 SAG (3) | TR, ms | 5000 | 2:25 |
TE, ms | 100 | ||
FOV, cm | 20×20 | ||
Matrix | 512×512 | ||
NAQ | 1 | ||
Spacing, mm | 3,3 | ||
ETL, ms | 9 | ||
T2 COR (3) | TR, ms | 5000 | 2:25 |
TE, ms | 100 | ||
FOV, cm | 20×20 | ||
Matrix | 512×512 | ||
NAQ | 1 | ||
Spacing, mm | 3,3 | ||
ETL, ms | 9 | ||
DWI (b=1000) | TR, ms | 6858 | 3:25 |
TE, ms | 100 | ||
FOV, cm | 30×30 | ||
Matrix | 256×256 | ||
NAQ | 5 | ||
Spacing, mm | 3 | ||
ETL, ms | 60 | ||
Т1 AX (5) | TR, ms | 5,5 | 0:15 |
TE, ms | 2,5 | ||
FOV, cm | 25×27 | ||
Matrix | 640×476 | ||
NAQ | 1 | ||
Spacing, mm | 2,5 | ||
Total scanning time (min) | 10:55 | ||
Notably, according to PI-RADS, T1-WI is not a mandatory sequence for bpMRI. Its inclusion in the protocol is based on the desire to provide an opportunity to assess both secondary damage to the lymph nodes and bone structures of the study area, and the presence of hemorrhagic changes in the tissues of the gland and seminal vesicles. The duration of T1-WI in the proposed configuration was 15 s, which does not significantly affect the total time of the study.
Quality control
The traditional approach of assessing the technical condition of an MRI involves scanning the phantom and calculating the quantitative characteristics of image quality. In this work, we used the standard control procedure for the developed protocol [9]. Based on the obtained images, the brightness inhomogeneity, resolution, nonlinearity, and measured slice thickness were calculated (Table 5).
The tomograph characteristics in the manufacturer’s documentation were taken as acceptable values. Notably, the frequently used signal-to-noise ratio parameter in this study was not determined because of the lack of a reference value for the protocol developed.
Clinical images
The resulting protocol provides a sufficiently high level of visualization quality. Fig. 5 presents images obtained using the optimized protocol. The total scanning time was less than 11 min.
Fig. 5. Images obtained using the accelerated protocol of biparametric magnetic resonance imaging. Patient with prostate changes consistent with PI-RADS 2: a) T2-WI, axial view; b, c) DWI and ICD.
DISCUSSION
PC is one of the major causes of death among men. For instance, PC ranks second in the structure of mortality from cancer in the USA [10] and third in European countries [11]. Despite the improvements in methods for diagnosing PC and monitoring of prostate-specific antigen, the incidence in Russia remains high [12]. Age, race (Negroid), and family history are currently established risk factors for PC, with the risk of the disease being higher in cases of PC diagnosed in close relatives at an early age or in the presence of several relatives with an established diagnosis [13].
BpMRI is a scanning protocol that includes only T2-WI and DWI with MCD maps. All patients with PI-RADS of 3 or higher and is suspected for malignancy are routinely biopsied [14]. Moreover, since such lesions require further attention (T2-WI and DWI are the main pulse sequences), the effect of dynamic contrast enhancement may not be decisive. As a result, bpMRI can be widely introduced into clinical practice. The use of such a protocol as a rapid noninvasive test for further routing of male patients in need of examination and those at low risk of clinically significant PC seems possible.
T. Tamada et al. [6] showed that bpMRI is comparable with mpMRI for the detection of clinically significant PC by PI-RADS v2.1. However, it is worth noting that diagnostic sensitivity was significantly higher with mpMRI than with bpMRI, while specificity was significantly higher with bpMRI than with mpMRI. Therefore, the use of bpMRI using the PI-RADS v2.1 protocol can help to avoid unnecessary biopsies. In their study, R.L. Sherrer et al. [14] showed that in patients with negative results in diagnostics of clinically significant PC by bpMRI, no tumor pathology was detected by mpMRI either. It is fair to say that, although bpMRI in the work by J.P. Zawaideh et al. [15] was comparable with mpMRI, multiparametric study revealed fewer lesions classified as PI-RADS 3 (8.3%) than bpMRI (17%), and fewer false positive results (11.4% vs. 18.9%), which provides a higher specificity (74% vs. 67%) of mpMRI.
For an adequate assessment of MR images by a radiologist, a number of factors are required, namely, proper patient preparation, the selection of a scanning mode depending on the final purpose of the study, as well as the selection of optimal scanning parameters. In addition, it is worth noting that diagnostics can be difficult (due to severe artifacts from intestinal motility or, e.g., hip implants) so that the study cannot be evaluated by PI-RADS by definition. Awareness of the patient’s history is also an important factor. Clinical and laboratory data, with the analysis of MR images, provide a more complete presentation of the patient’s condition.
Speaking of optimizing the work of departments, the issues of acceleration of prostate scanning protocols are also raised in the international literature. To reduce the time and decrease the cost of the study, M. van der Leest et al. [16] proposed a protocol containing only T2-WI, DWI, and MDC for diagnostics of clinically significant pc. The paper demonstrates that unnecessary biopsy was avoided in 47% of patients using the accelerated bpMRI protocol, while conventional bpMRI and mpMRI protocols required biopsy in 49% of cases. The authors reported that accelerated bpMRI can be performed in 8 min, reducing direct costs by more than half (54%) compared with mpMRI and by 37% compared with conventional bpMRI. The inter-expert agreement planned by the study was 90% for rapid bpMRI and 93% for conventional bpMRI [16]. Simultaneously, according to the results of A. Stanzione et al. [17], the diagnostic accuracy of the accelerated bpMRI protocol was the lowest (83%) in contrast to the bpMRI and mpMRI protocols (86% and 87%, respectively). The authors concluded that such a protocol is not applicable for this purpose and demonstrated the results that the interpretation of studies depends directly on the experience of radiologists.
R. Engels et al. [8] conducted a large-scale analysis, which resulted in the summation of the minimum recommended technical parameters for mpMRI in the form of a table, where the total scanning time on a biparametric protocol using 1.5-T tomographs was more than 13 min. In our study, we managed to achieve shortening of the scanning protocol time, for example, when using the recommended PI-RADS v2.1 pulse sequences for bpMRI, and the duration of our protocol was 10 min 40 sec. We also proposed to use additionally T1-WI in the axial projection with duration of 15 s, with the total scanning time of 10 min 55 sec.
The protocol developed by us is the stage 1 of our work on the feasibility of using MRI as a screening method for PC. More evidence must confirm this hypothesis, which gives further scope for future research. Only three clinical cases from three different MHD HOs are presented, demonstrating the heterogeneity of the prostate scanning protocols. Simultaneously, note that the protocol was developed for one MRI model (Excelart Vantage 1.5 T), for which the quality parameters were calculated. The protocol, certainly, requires adaptation and testing on other models of tomographs, followed by evaluation of the results.
The protocol requires further study on the diagnostic accuracy for the detection of PC, such as using textural analysis, which is a subject of increased interest in diagnostics of PC [18], to compare with the results of histopathological conclusions. Therefore, the next step will be a statistical comparison of findings obtained using standard prostate scanning protocols for different HO MHD and an optimized protocol.
The protocol should be subjected to further evaluation of the total time expenditures and economic feasibility in general.
CONCLUSION
The sets of pulse sequences depend directly on the aim of the study, as well as the individual preferences of radiologists and clinicians of particular HOs. The established, “familiar” scanning protocols for a particular department of radiation diagnostics because of the lack of standardized, unified protocols pose a problem. The procedure in each specific HO is performed according to different protocols using a set of different pulse sequences and technical characteristics, which influences, among other things, on the study duration. This is because scanning that fully meets the recommendations of PI-RADS v2.1 is not always possible under the conditions of the HO MHD for diagnostics of PC. We offer an optimized bpMRI protocol. The technical characteristics of the proposed protocol are as close as possible to the PI-RADS standards, while the scanning time is less than 11 min, which can certainly be essential in optimizing the work of radiology departments under high load conditions.
A standardized protocol using acquired MR images has the potential for further training and implementation of artificial intelligence in male pelvic examination programs through imaging techniques.
ADDINIONAL INFORMATION
Funding source. This article was prepared as part of research (№ ЕГИСУ: АААА-А21-121012290079-2) under the Program of the Moscow Healthcare Department “Scientific Support of the Capital’s Healthcare” for 2020-2022.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Yu.A. Vasilev — study design, MRI protocol optimization, manuscript revising, advisory support, L.R. Abuladze — study design, data analysis, manuscript drafting; D.S. Semenov — study design, data analysis, manuscript drafting, technical audit; O.Yu. Panina — manuscript revising, advisory support.
About the authors
Liya R. Abuladze
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: l.abuladze@npcmr.ru
ORCID iD: 0000-0001-6745-1672
SPIN-code: 5640-9989
Russian Federation, Moscow
Dmitriy S. Semenov
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: d.semenov@npcmr.ru
ORCID iD: 0000-0002-4293-2514
SPIN-code: 2278-7290
Scopus Author ID: 57213154475
ResearcherId: P-5228-2017
Russian Federation, Moscow
Olga Y. Panina
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies; City Clinical Oncological Hospital No. 1; Moscow State University of Medicine and Dentistry named after A.I. Evdokimov
Email: olgayurpanina@gmail.com
ORCID iD: 0000-0002-8684-775X
SPIN-code: 5504-8136
ResearcherId: AAG-6447-2020
Russian Federation, Moscow; Moscow; Moscow
Yuriy A. Vasilev
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Author for correspondence.
Email: dr.vasilev@me.com
ORCID iD: 0000-0002-0208-5218
SPIN-code: 4458-5608
Russian Federation, Moscow
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. doi: 10.1007/s00330-011-2377-y
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging ― reporting and data system: 2015, version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052
- Park SY, Jung DC, Oh YT, et al. Prostate cancer: PI-RADS version 2 helps preoperatively predict clinically significant cancers. Radiology. 2016;280(1):108–116. doi: 10.1148/radiol.16151133.
- Israël B, van der Leest M, Sedelaar M, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. part 2: interpretation. Eur Urol. 2020;77(4):469–480. doi: 10.1016/j.eururo.2019.10.024
- Tamada T, Kido A, Yamamoto A, et al. Comparison of biparametric and multiparametric mri for clinically significant prostate cancer detection with pi-rads version 2.1. J Magn Reson Imaging. 2021;53(1):283–291. doi: 10.1002/jmri.27283
- Patent RUS 208239 U1. Semenov DS, Petryaykin AV, Vasiliev YuA, et al. Phantom device for configuring protocols of magnetic resonance imaging of the prostate gland in patients with metal structures of the hip joint. (In Russ). Available from: https://www.elibrary.ru/item.asp?id=47429681. Accessed: 15.03.2022.
- Engels RR, Israël B, Padhani AR, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 1: acquisition. Eur Urol. 2020;77(4):457–468. doi: 10.1016/j.eururo.2019.09.021
- Methodology for monitoring the parameters and characteristics of magnetic resonance tomographs under operating conditions Methodological recommendations No. 17 (approved 10.09.2011). (In Russ). Available from: https://docs.cntd.ru/document/456079947. Accessed: 15.03.2022.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005
- Malignant neoplasms in Russia in 2020 (morbidity and mortality). Ed. by A.D. Kaprin, V.V. Starinsky, A.O. Shakhzadova. Moscow; 2021. 252 p. (In Russ).
- Patel AR, Klein EA. Risk factors for prostate cancer. Nat Clin Pract Urol. 2009;6(2):87–95. doi: 10.1038/ncpuro1290
- Sherrer RL, Glaser ZA, Gordetsky JB, et al. Comparison of biparametric MRI to full multiparametric MRI for detection of clinically significant prostate cancer. Prostate Cancer Prostatic Dis. 2019;22(2):331–336. doi: 10.1038/s41391-018-0107-0
- Zawaideh JP, Sala E, Shaida N, et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: assessment of contrast benefit in clinical practice. Eur Radiol. 2020;30(7):4039–4049. doi: 10.1007/s00330-020-06782-0
- Van der Leest M, Israël B, Cornel EB, et al. High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in biopsy-naïve men: the next step in magnetic resonance imaging accessibility. Eur Urol. 2019;76(5):574–581. doi: 10.1016/j.eururo.2019.05.029
- Stanzione A, Ponsiglione A, Cuocolo R, et al. Abbreviated protocols versus multiparametric mri for assessment of extraprostatic extension in prostatic carcinoma: A multireader study. Anticancer Res. 2019;39(8):4449–4454. doi: 10.21873/anticanres.13617
- Gelezhe PB, Blokhin IA, Semenov SS, et al. Radiomics of magnetic resonance imaging in prostate cancer: what is currently known? Digital Diagnostics. 2021;2(4):441–452. (In Russ).
Supplementary files