Latent course of Crohn’s disease: the role of tomographic imaging in diagnosis
- Authors: Shumskaya Y.F.1,2, Nefedova T.S.1, Akhmedzyanova D.A.1, Blokhin I.A.2, Mnatsakanyan M.G.1
-
Affiliations:
- The First Sechenov Moscow State Medical University (Sechenov University)
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- Issue: Vol 3, No 4 (2022)
- Pages: 394-402
- Section: Case reports
- Submitted: 16.09.2022
- Accepted: 11.10.2022
- Published: 30.12.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/110952
- DOI: https://doi.org/10.17816/DD110952
- ID: 110952
Cite item
Abstract
Crohn’s disease with localization in the upper gastrointestinal tract, terminal ileum, or colon is diagnosed based on visualization of the lesion area using endoscopic methods and histological examination. In cases of damage to the small intestine, when endoscopy methods are not informative enough and the use of videocapsular endoscopy has a number of contraindications, it is advised to use radiation diagnostic methods, such as multispiral computed tomography and/or magnetic resonance enterography, to make a diagnosis.
We present a clinical case of ambiguous clinical manifestations of Crohn’s disease with small intestine and rectal involvement. Tomographic imaging was used to confirm the diagnosis. A 44-year-old patient presented with complaints of non-pronounced abdominal pain, dyspepsia. The lab panel showed indirect signs of malabsorption, an increase in fecal calprotectin. An endoscopic examination with histological verification revealed a picture of proctitis. After performing computed tomography and/or magnetic resonance enterography multiple lesions of the small intestine were revealed. This clinical case demonstrates an atypical clinical picture of Crohn’s disease with jejunal, iliac, and rectal lesions.
The patient had no characteristic complaints; the results of endoscopic and morphological studies were not informative. Imaging by means of computed and magnetic resonance tomography has played a crucial role in the diagnosis and successful treatment.
Keywords
Full Text
BACKGROUND
Because of its systemic nature, Crohn’s disease can affect not only the gastrointestinal tract but also the musculoskeletal or respiratory system, organs of vision, and skin [1-4]. Thus, Crohn’s disease frequently draws the attention of doctors from various specialties. The possible polymorphism of complaints, particularly at the time of disease onset, with extraintestinal symptoms or rare complaints in the latent stages make the diagnosis of this disease extremely difficult [5, 6] and prevents the timely prescription of adequate therapy.
In classic cases, Crohn’s disease is diagnosed through endoscopic visualization of the affected area, which is only possible when the disease is localized in the upper gastrointestinal tract, terminal ileum, or colon. However, when the small intestine is affected, endoscopic methods become uninformative, whereas video capsule endoscopy has contraindications, making it difficult to use in clinical practice [7]. Thus, radiation diagnostic methods, such as multislice computed tomography (MSCT) and/or magnetic resonance (MR) enterography, should be used for diagnosis [8, 9].
We present the case of a patient with Crohn’s disease with inapparent clinical manifestations and damage to the small intestine and rectum whose diagnosis was confirmed using radiation diagnostic methods.
CLINICAL CASE
Patient
Patient D., 44 years old, was admitted to the gastroenterology department with complaints of umbilical discomfort, bloating, and fullness in the epigastrium and umbilical region that appears 30–60 minutes after a meal. In 2017, the patient noticed a feeling of heaviness in the abdomen, gaseous eructation, and episodes of heartburn after consuming food, and lost 15 kg in 2 years with no dietary changes. The patient underwent outpatient examination, which revealed no gastrointestinal pathology. Abdominal ultrasound revealed no abnormalities; oesophagogastroduodenoscopy showed superficial gastritis not associated with Helicobacter pylori infection (negative rapid urease test); and colonoscopy showed no organic pathology. The condition was classified as the sphincter of Oddi functional disorder and the patient was treated with rabeprazole and hymecromone but without any significant effect. Episodic abdominal pain (once in every several months) persisted. The patient was hospitalized for examination for the aforementioned complaints.
The patient’s condition at the time of admission was satisfactory. Their physique was asthenic, with a body mass index of 20.02 kg/m2. Body temperature on admission was 36.5°C; the skin was pale; and the abdomen was visually symmetrical and tender on palpation in the umbilical, right mesogastric, and right iliac regions. The bowel movements were normal without pathological admixtures.
Laboratory and instrumental findings
According to laboratory findings during hospitalization, hemoglobin level decreased from 137.2 to 123 g/L (normal range: 132–180 g/L), serum iron level decreased to 10.4 µmol/L (normal range: 12.5–32.2 µmol/L), total protein level decreased to 63 g/L (normal range: 66–83 g/L), fecal occult blood test was positive, and fecal calprotectin level was elevated to 389 µg/g (normal value: up to 50 µg/g). All other parameters in complete blood count, blood chemistry, coagulogram, urinalysis, and stool test remained within the normal range.
Oesophagogastroduodenoscopy revealed no signs of upper gastrointestinal damage.
Colonoscopy revealed endoscopic signs of proctitis: swelling of the rectal mucosa, multiple hemorrhages, and a smoothened vascular pattern. A biopsy was performed.
Histological findings revealed the following: preserved architectonics of the rectal mucosa, dense uniform lymphoplasmacytic infiltration in the deep mucosa with an abundance of eosinophilic leukocytes, and hyperplastic lymphoid follicles with proliferative centers in several fragments.
These findings did not indicate a uniform pattern. Given the indirect signs of malabsorption syndrome (low hemoglobin, serum iron, and total protein), the small intestine was examined.
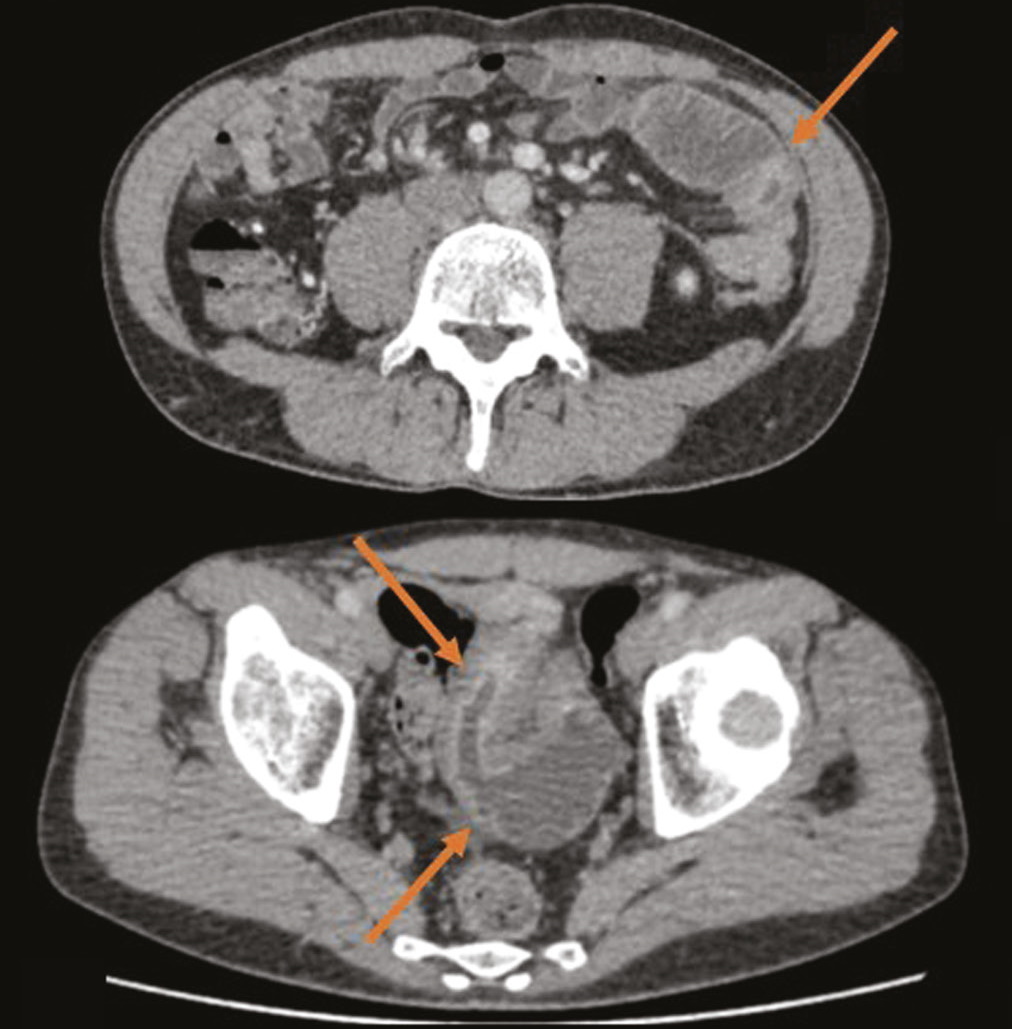
Abdominal MSCT with intravenous contrast was performed after the oral administration of 1 L of macrogol solution. The jejunum and ileum walls were locally thickened with inactive contrast agent accumulation. Approximately 5 cm of the wall of the distal jejunum transitioning into the ileum was markedly thickened up to 17 mm, with more active contrast agent accumulation, including in the mucosa (Fig. 1). Regional adipose tissue was infiltrated with the formation of liquid zones along the midline between the loops in the small pelvis; and regional lymph nodes as large as 9 mm showed active accumulation of the contrast agent.
Fig. 1. Abdominal multislice computed tomography with intravenous contrast, axial plane: a) substantial narrowing of the intestinal lumen and thickening of the wall with active contrast agent accumulation (arrow); b) dilated loop of the small intestine with an unevenly thickened wall (arrow); c) dilation and narrowing of the intestinal lumen is visible; additionally, of interest is the intestinal mucosa, which actively accumulates the contrast agent (arrows); d) the area of fluid accumulation between the loops in the small pelvis is marked red.
To determine the extent and volume of the lesion, MR enterography was performed after administering 1.2 L of mannitol solution orally. Four local areas of uneven wall thickening were noted in the small intestine: thickening of up to 8 mm over 25 mm with narrowing of the lumen to 5 mm, thickening of up to 12 mm over ~90 mm with narrowing of the lumen to 3 mm, thickening of up to 10 mm over 160 mm with narrowing of the lumen to 3 mm, and thickening of up to 9 mm over ~32 mm with narrowing of the lumen to 3 mm (Figs. 2 and 3). These areas of the small intestine actively accumulated the contrast agent and showed signs of limited diffusion on diffusion-weighted imaging. Because the lesions resembled those seen in intestinal tuberculosis, an immunodiagnosis of tuberculosis infection (interferon gamma release assays, T-SPOT.TB, with a negative result) and chest MSCT (with no pathology) were performed.
Fig. 2. Magnetic resonance enterography, axial plane: the arrows show thickened areas of the small intestine.
Fig. 3. Magnetic resonance enterography, coronal plane: the arrows show areas of thickening of the long sections of the small intestine walls.
Diagnosis and treatment
Considering the “kangaroo jumping” type of gastrointestinal lesion discovered during the examination along with the findings of colonoscopy and histological examinations, the patient was diagnosed with Crohn’s disease with strictures and lesions of the small intestine and rectum.
To relive the condition, prednisolone was intravenously administered at a dose of 120 mg/day, with a gradual decrease in the dose and a switch to oral methylprednisolone at a dose of 8 mg/day. In addition, the patient received mesalazine rectally at a dose of 2 g/day. To maintain remission, a genetically engineered biological drug (infliximab) and azathioprine were chosen because of the atypical localization of the process, the extent of the lesion, and the high activity of the disease.
During treatment, the patient’s condition improved; there were no complaints of abdominal pain and dyspepsia. At a follow-up examination after 3 months, the level of fecal calprotectin was within the normal range, and blood chemistry showed no signs of malabsorption. The patient had gained approximately 5 kg of weight.
DISCUSSION
The clinical case presented here shows an unusual clinical picture of Crohn’s disease, exhibiting damage to the jejunum, ileum, and rectum. In the absence of specific complaints and with insufficient endoscopic and morphological findings, tomographic radiation diagnostics played a critical role in establishing the diagnosis.
According to the clinical guidelines for the diagnosis and treatment of Crohn’s disease, MSCT and MR enterography are only used to rule out small intestine strictures before performing video capsule endoscopy [10]. However, published foreign literature demonstrates the inherent value of radiation tomography methods for diagnosing Crohn’s disease, including a consensus on their use [11].
Chavoshi et al. [12] conducted a systematic review and found that the sensitivity and specificity of MR enterography in detecting lesions of the small intestine in Crohn’s disease were 80%–88% and 81%–91%, respectively, which are sufficient to make MR enterography a popular method for diagnosing pathologies associated with Crohn’s disease in the small intestine.
According to Park et al. [13] the data obtained using abdominal MSCT were significantly correlated with the Crohn’s disease activity index and C-reactive protein level (p < 0.05). AUC was 0.85 when performing ROC analysis on MSCT data to predict disease activity. Sensitivity and negative predictive values were 95% and 94%, respectively, with a cutoff value of 0.8.
Publications in the Russian literature describe the diagnostic value of tomography. For example, Dubrova and Stashuk [14] emphasized the importance of using these methods in conjunction with endoscopic methods in the diagnosis of Crohn’s disease. Kurilo et al. [15] demonstrated the exceptional importance of MSCT enterography in a series of clinical cases, obtaining data on the localization and extent of pathological changes, process activity, and presence of extraintestinal complications. These data allowed determining the tactics for managing patients with complicated Crohn’s disease (such as with perforation of the ileum in one case and decompensated stenosis of the descending colon in another).
Our clinical case also demonstrates the importance of using tomographic radiation methods for the diagnosis of Crohn’s disease with small intestine lesions to determine the activity and extent of the lesion. The information obtained via enterography was crucial for diagnosing the disease and deciding the treatment tactics.
CONCLUSION
Crohn’s disease does not always present with pronounced clinical symptoms. Routine examinations, including endoscopies, are often insufficient to diagnose small bowel disease. Tomographic methods (CT and MR enterography) are highly informative and accurate and allow the visualization of the small intestine when assessing the volume and activity of the lesion.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Yuliya F. Shumskaya — conceptualization, data curation, investigation, writing the original draft, revising and editing the manuscript; Tamara S. Nefedova, Dina A. Akhmedzyanova — conceptualization, data curation, investigation; Ivan A. Blokhin — writing, revising and editing the manuscript; Marina G. Mnatsakanyan — conceptualization, investigation, and supervision.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics Journal.
About the authors
Yuliya F. Shumskaya
The First Sechenov Moscow State Medical University (Sechenov University); Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: yu.shumskaia@npcmr.ru
ORCID iD: 0000-0002-8521-4045
Russian Federation, Moscow; Moscow
Tamara S. Nefedova
The First Sechenov Moscow State Medical University (Sechenov University)
Email: prosto.toma.22@gmail.com
ORCID iD: 0000-0002-6718-8701
SPIN-code: 3097-4977
Russian Federation, Moscow
Dina A. Akhmedzyanova
The First Sechenov Moscow State Medical University (Sechenov University)
Email: dina_akhm@mail.ru
ORCID iD: 0000-0001-7705-9754
SPIN-code: 6983-5991
Russian Federation, Moscow
Ivan A. Blokhin
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: i.blokhin@npcmr.ru
ORCID iD: 0000-0002-2681-9378
SPIN-code: 3306-1387
Russian Federation, Moscow
Marina G. Mnatsakanyan
The First Sechenov Moscow State Medical University (Sechenov University)
Author for correspondence.
Email: mnatsakanyan08@mail.ru
ORCID iD: 0000-0001-9337-7453
SPIN-code: 2015-1822
MD, Dr. Sci. (Med.)
Russian Federation, MoscowReferences
- Ainouche A, Durot C, Soyer P, et al. Unusual intestinal and extra intestinal findings in Crohn’s disease seen on abdominal computed tomography and magnetic resonance enterography. Clin Imaging. 2020;59(1):30–38. doi: 10.1016/j.clinimag.2019.04.010
- Jumani L, Kataria D, Ahmed MU, et al. The Spectrum of extra-intestinal manifestation of Crohn’s disease. Cureus. 2020;12(2):e6928. doi: 10.7759/cureus.6928
- Amado C, Ferreira PG. Pleuroparenchymal fibroelastosis associated with Crohn’s disease: a new aetiology? Eur J Case Rep Intern Med. 2020;7(12):002017. doi: 10.12890/2020_002017
- Dodd EM, Howard JR, Dulaney ED, et al. Pyodermatitis-pyostomatitis vegetans associated with asymptomatic inflammatory bowel disease. Int J Dermatol. 2017;56(12):1457–1459. doi: 10.1111/ijd.13640
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21(3):211–214. doi: 10.1111/j.1365-2230.1996.tb00065.x
- Dore M, Junco TP, Galán SA, et al. Pitfalls in diagnosis of early-onset inflammatory bowel disease. Eur J Pediatr Surg. 2018;28(1):39–43. doi: 10.1055/s-0037-1604428
- Cave DR, Hakimian S, Patel K. Current controversies concerning capsule endoscopy. Dig Dis Sci. 2019;64(11):3040–3047. doi: 10.1007/s10620-019-05791-4
- Minordi LM, Larosa L, Papa A, et al. Assessment of Crohn’s disease activity: magnetic resonance enterography in comparison with clinical and endoscopic evaluations. J Gastrointestin Liver Dis. 2019;28:213–224. doi: 10.15403/jgld-183
- Hokama A, Kinjo T, Fujita J. Growing role of magnetic resonance enterography in the management of Crohn disease. Pol Arch Intern Med. 2020;130(9):724–725. doi: 10.20452/pamw.15628
- Clinical guidelines for the diagnosis and treatment of Crohn’s disease in adults (project). Koloproktologiya. 2020;19(2):8–38. (In Russ). doi: 10.33878/2073-7556-2020-19-2-8-38
- Bruining DH, Zimmermann EM, Loftus EV, et al; Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286(3):776–799. doi: 10.1148/radiol.2018171737
- Chavoshi M, Mirshahvalad SA, Kasaeian A, et al. Diagnostic accuracy of magnetic resonance enterography in the evaluation of colonic abnormalities in Crohn’s disease: a systematic review and meta-analysis. Acad Radiol. 2021;28(Suppl 1):S192–S202. doi: 10.1016/j.acra.2021.02.022
- Park EK, Han NY, Park BJ, et al. Value of computerized tomography enterography in predicting Crohn’s disease activity: correlation with Crohn’s disease activity index and C-reactive protein. Iran J Radiol. 2016;13(4):e34301. doi: 10.5812/iranjradiol.34301
- Dubrova SE, Stashuk GA. Possibilities of radiation methods in the diagnosis of inflammatory bowel diseases. Al’manakh klinicheskoi meditsiny. 2016;44(6):757–769. (In Russ). doi: 10.18786/2072-0505-2016-44-6-757-769
- Kurilo DP, Savchenko MI, Soloviev IA, et al. Possibilities of computed tomography angiography in the diagnosis of complicated forms of Crohn’s disease. Bulletin of the Russian military medical academy. 2020;(1):60–65. (In Russ).
Supplementary files