Тerminology of rectal cancer: consensus agreement of the expert working group
- Authors: Berezovskaya T.P.1, Rubtsova N.A.2, Sinitsyn V.E.3, Zarodnyuk I.V.4, Nudnov N.V.5, Mishchenko A.V.6, Trubacheva Y.L.4, Bergen T.A.7, Grishko P.Y.6, Balyasnikova S.S.8, Dayneko Y.A.1, Ryjkova D.V.9, Hodzhibekova M.M.2, Rucheva N.A.10, Turin I.E.8, Achkasov S.I.4, Nevolskikh A.A.1, Gordeev S.S.8, Droshneva I.V.2
-
Affiliations:
- A.F. Tsyba Medical Radiological Research Center ― branch National Medical Research Radiological Center
- P.A. Herzen Moscow Research Oncological Institute ― branch National Medical Research Radiological Center
- Lomonosov Moscow State University
- State Scientific Centre of Coloproctology
- Russian Scientific Center of Roentgenoradiology
- N.N. Petrov National Medical Research Centre of Oncology
- E. Meshalkin National Medical Research Center
- N.N. Blokhin National Medical Research Center of Oncology
- Almazov National Medical Research Centre
- V.I. Shumakov National Medical Research Center of Transplantology and Artificial Organs
- Issue: Vol 4, No 3 (2023)
- Pages: 306-321
- Section: Clinical Practice Guidelines
- Submitted: 03.07.2023
- Accepted: 10.07.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/529668
- DOI: https://doi.org/10.17816/DD529668
- ID: 529668
Cite item
Abstract
Unified terminology is a necessary condition for successful interdisciplinary communication within the field of oncology. The variety of anatomical, pathomorphological, and clinical terms used in rectal cancer is often accompanied by their ambiguous interpretation both in domestic and foreign scientific literature. This not only complicates the interaction between specialists, but also complicates the comparison of the results of rectal cancer treatment obtained in different medical institutions.
Based on the analysis of recent domestic and international scientific and methodological literature on rectal cancer, the key terms used in the diagnosis and treatment planning of rectal cancer were selected, followed by a two-time online discussion of their interpretations by experts from the Russian Society of Radiologists and Therapeutic Radiation Oncologists, the Association of Oncologists of Russia, and the Russian Association of Therapeutic Radiation Oncologists until reaching consensus (≥80%) of experts on all items. Terms that fail to attain consensus were excluded in the final list.
A list of anatomical, pathomorphological, and clinical terms used in the diagnosis, staging, and treatment planning of rectal cancer has been compiled and, based on expert consensus, their interpretation has been determined.
A lexicon recommended in the description and formulation of the conclusion of diagnostic studies in patients with rectal cancer is proposed.
Full Text
List of abbreviations
AOR — Association of Oncologists of Russia
ICD-10 — International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
MRI — Magnetic resonance imaging
RATRO — Russian Association of Therapeutic Radiation Oncologists
RORR — Russian Society of Radiologists and Radiologists
T2-WI — T2-weighted image (image acquisition mode in magnetic resonance imaging)
CRM — Circumferential resection margin
AJCC-TNM8 — National Validation of the 8th American Joint Committee on Cancer Staging System
TNM — International classification of stages of malignant neoplasms (tumor, nodus, and metastasis)
TRG — Tumor regression grading
INTRODUCTION
Uniform terminology in describing radiological examinations and formulating conclusions for patients with rectal cancer is crucial for ensuring adequate understanding among the treating physician and all members of the multidisciplinary medical team. Currently, magnetic resonance imaging (MRI) plays a crucial role in staging, supplemented by transrectal ultrasound in the early stages of rectal cancer. Staging involves assessing various anatomical and pathological factors that influence rectal cancer treatment planning. It is essential for all specialists to be aware of relevant terms and their clear interpretation for effective communication for the benefit of patients and comparison of rectal cancer treatment outcomes across different healthcare institutions.
CONSENSUS GUIDELINES ON TERMINOLOGY AND INTERPRETATION OF RECTAL CANCER IMAGING RESULTS
Procedure for a working group to create national recommendations on a unified terminology for rectal cancer diagnostics
To accomplish this goal, a working group (WG) comprising experts from the Russian Society of Radiologists and Radiologists (RORR) was established. The group represented 10 leading healthcare institutions in the Russian Federation providing specialized care to patients with rectal cancer. Additionally, experts from the Association of Oncologists of Russia (AOR) and the Russian Association of Therapeutic Radiation Oncologists (RATRO) were involved in developing the current clinical guidelines for rectal cancer. The group included 15 diagnostic radiology specialists, 3 surgical oncologists, and a radiology oncologist.
Three WG members, who are authors of this paper (BTP, MAV, and GPJ) searched the PubMed, Medline, and eLibrary databases for literature about staging, prevalence assessment, treatment planning in rectal cancer, and evaluation of effectiveness of neoadjuvant therapy from 2007 to 2023, with extraction of the main terms and their interpretations. The list of terms was sent to all WG members for review, followed by two online discussions until an expert consensus (≥80%) was reached regarding the interpretation of each term. Terms that did not reach a consensus opinion (low-grade rectal cancer, early rectal cancer, and tumor regrowth) were excluded from this paper. The final version of the manuscript was sent to all WG members and received their approval.
A consensus list of recommended terms of staging, extent assessment, and treatment planning in rectal cancer and their definitions for use in medical diagnostic reports (primarily for MRI) is given below.
List of terms and their definitions agreed by expert consensus
Terms to assess prevalence and location of rectal cancer
Rectal cancer is a malignant tumor that develops from rectal epithelial cells, typically exhibiting adenocarcinoma structure and localized within 15 cm from the anus (ICD-10 code: C20) [1]. Tumors with a lower pole located above this level are classified as malignant neoplasms of the rectosigmoid junction (ICD-10 code: C19). Tumors with the histological structure of squamous cell carcinoma, localized in the anal canal, are classified as malignant neoplasms of the anus and anal canal (ICD-10 code: C21). Squamous cell carcinoma of the anal canal can spread above the anorectal junction and involve the rectal ampulla, while rectal cancer with the structure of adenocarcinoma can spread into the anal canal or have a predominant localization there. In such cases, the histological type of malignant neoplasms determines not only the approach to TNM classification (rectal cancer/anal cancer) but also the choice of treatment method.
Some anatomical structures of the pelvis, rectum, and anal canal play an important role in the primary staging of rectal cancer. Awareness of these structures and the ability to recognize them on images is critical for accurate characterization of the primary tumor [2–5].
Surgical anal canal: For transrectal ultrasound and MRI, the lower border of the anal canal is considered to be the distal edge of the internal sphincter. For transrectal ultrasound, the upper border of the internal sphincter is considered to be the upper border. For MRI, the anorectal junction is considered.
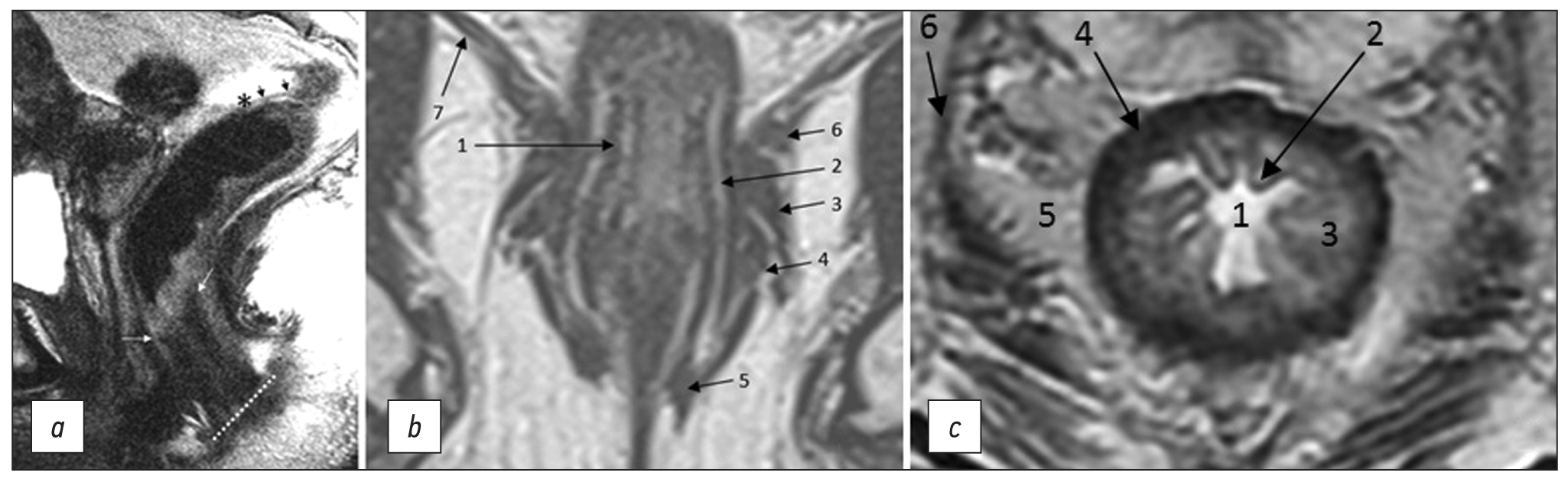
The anal margin refers to the anocutaneous line, the junction of the anoderm with the perianal skin, corresponding to the distal edge of the internal sphincter of the anal canal/intersphincteric groove on sagittal MRI (Fig. 1). From this level, the distance to the lower edge of the tumor is measured on sagittal T2-weighted images along the center of the lumen of the anal canal and rectum [6].
The anorectal junction is the connection between the anal canal and the rectum, corresponding to the upper edge of the puborectalis muscle, clearly defined on T2-WI in the coronal plane, or the anorectal angle, defined on T2-WI in the sagittal plane (Fig. 1). The distance from the anorectal junction to the inferior margin of the tumor is important for surgery planning and should be indicated in the diagnostic report [6].
The dentate line is the upper limit of the anatomical anal canal, which is shorter than the surgical one. The location of the dentate line approximately corresponds to the middle of the internal sphincter. It is not visualized by MRI.
The anal sphincter complex includes the internal and external anal sphincters and the puborectalis muscle (Fig. 1).
Fig. 1. MRI anatomy of the rectum on T2-WI. (a) Sagittal plane: anal edge (intersphincteric groove; dotted line); anorectal junction (angle) at the level of the upper border of the internal sphincter of the anal canal (white arrows); transitional fold of the peritoneum at the lower point of attachment of the pelvic visceral peritoneum to the rectal wall (asterisk); peritonealized part of the rectum (black arrows). (b) Coronal plane: 1, internal sphincter of the anal canal; 2, intersphincteric space; 3, deep portion of the external sphincter; 4, superficial portion of the external sphincter; 5, subcutaneous portion of the external sphincter; 6, puborectalis muscle; 7, elevator muscle of anus (levator ani). (c) Axial plane: 1, intestinal lumen; 2, mucous membrane; 3, submucosal layer; 4, muscle layer; 5, mesorectal tissue; 6 mesorectal fascia.
The internal anal sphincter is a continuation of the internal circular muscle layer of the rectum, comprising smooth muscle tissue. On MRI, it is determined by a significant thickening of the intrinsic muscle layer of the wall at the level of the anal canal (Fig. 1). The signal from the internal sphincter on T2-weighted images is slightly higher than that of the external sphincter, and with contrast enhancement, it appears more intense.
The external anal sphincter is a striated muscle, which is a continuation of the puborectal muscle, divided into three layers, such as the subcutaneous circular layer, superficial ellipsoidal layer, connected to the coccyx, and deep layer, closely connected with the puborectalis muscle (Fig. 1).
The intersphincteric space is a connective-cellular tissue space that separates internal and external anal sphincters and is characterized by a high signal on T2-WI (Fig. 1).
Mesorectal fascia is a thin fascial sheath that limits the rectum and the surrounding fatty tissue. On T2-WI, it appears as a hypointense circular line (Fig. 1). In men, the mesorectal fascia in front merges with the Denonvilliers’ fascia, while in women, it merges with the rectovaginal fascia (septum). At the back, it connects with the presacral fascia; it completely surrounds the rectum only to the level of the transitional fold of the peritoneum with its lateral and posterior parts above and only the posterior rectum at the level of the upper ampullary. Caudally, the mesorectal fascia passes into the intersphincteric space.
The muscular layer of the rectum consists of an inner circular and outer longitudinal layer, which are defined as a single hypointense layer on T2-weighted MRI images, limited internally by a hyperintense submucosal layer and externally by hyperintense mesorectal tissue (Fig. 1).
The elevator muscle of the anus (m. levator ani) is a muscle complex (Fig. 1), consisting of the puborectalis, pubococcygeus, iliococcygeus, and anal-coccygeal fibrous muscles and anococcygeal ligament. Tumors infiltrating the elevator muscle of anus are classified as T4b.
The transitional fold of peritoneum is formed at the point of transition of the peritoneum from the pelvic organs to the rectal wall, with the lower point of attachment along the anterior wall of the intestine and obliquely going up the side walls. It separates the peritonized and non-peritonized parts of the rectum. On T2-WI, it appears as a hypointense line, displaying a V-shape in the axial plane, and passes from the apex of the seminal vesicles (in men) or from the body of the uterus (in women) in the sagittal plane (Fig. 1). Lymphatic drainage from tumors located above the transitional fold of the peritoneum mainly occurs through the upper rectal and lower mesenteric lymph nodes. Tumors below the transitional fold of the peritoneum can drain through the internal iliac and obturator lymph nodes. When describing MRI findings, it is recommended to indicate the position of the tumor relative to the transitional fold of the peritoneum (completely below/crosses/completely above) [6].
The presacral space is a fibrous space delimited in front by the presacral fascia (the posterior part of the parietal layer of the fascia of the pelvis). It contains presacral veins and plexuses.
The rectal mucosa is the innermost, thin layer of the rectal wall. When visualized, it has a hypointense signal on T2-weighted images due to the lamina propria (Fig. 1).
Terms related to primary staging of rectal cancer
For primary staging of rectal cancer, MRI is the preferred diagnostic method. For the initial stages of rectal cancer, transrectal ultrasound is recommended [1, 3, 7–9]. Currently, staging is carried out according to the TNM classification of the Union for International Cancer Control (UICC; 8th ed., 2017) [10].
Below are some terms that, along with the “T” category, are important for characterizing the primary tumor [2, 4, 5].
The depth of extramural invasion is the maximum distance from the outer edge of the muscular layer of the wall at the base of the extramural component of the primary tumor to its outer edge, as observed on high-resolution T2-WI oriented perpendicular to the intestinal wall at the level of the tumor (Fig. 2). The depth of extramural invasion is used to determine the substage of T3 tumors.
Fig. 2. Circular border (edge) of rectal resection during total mesorectumectomy. (a) Diagram showing extramural growth of the tumor (green line); mesorectal fascia (yellow line); circular border (edge) of resection (red line); distance from the tumor to the mesorectal fascia (double black arrow); distance from the tumor to the circular border (edge) of resection (double red arrow). (b) T2-weighted images in the coronal plane of the tumor of the lower ampullary part of the rectum with extramural vascular invasion and deposit at the level of axial T2-weighted images. (c) Upper axial section corresponds to the level of the deposit involving the mesorectal fascia (black arrows), extramesorectal lymph node (dotted arrow). The lower axial section corresponds to the level of extramural vascular invasion. The depth of extramural invasion (a double white arrow). The distance from the tumor to the elevator muscle of anus (a double black arrow).
Category T according to the TNM system. Category T, established based on the examinations of a primary patient with rectal cancer, is called clinical and is denoted by the prefix “c” (cT). If radiological examination methods were used for staging, then the prefix “i” (iT) is used.
T1: Tumor has grown into the submucosa. Subclassification of T1 tumors according to Kikuchi [11]: T1sm1: depth of submucosa invasion up to 1/3; T1sm2: depth of submucosa invasion er up to 2/3; T1sm3: complete tumor invasion of the entire submucosal layer. Transrectal ultrasound is preferred to evaluate T1 tumors.
T2: Tumor has grown into the muscle layer. Transrectal ultrasound is reported to be more accurate in diagnosing T1/T2 tumors (sensitivity 94%, specificity 86%) compared with MRI (sensitivity 94%, specificity 70%) but less accurate in determining lymph node status [12].
T3: Tumor has grown through the muscularis propria and into the subserosa or non-peritoneal peri-intestinal tissue (T3 tumors are divided into the following substages: T3a <1mm; T3b 1–5mm; T3c 5–15mm; T4d >15mm). To assess extramural tumor growth, it is recommended to use high-resolution T2-WI perpendicular to the bowel wall at the level of the tumor.
T4: Tumor invades the serosa/peritoneum of the pelvis (T4a) or surrounding organs and tissues (T4b), including pelvic organs (uterus, ovaries, vagina, prostate, seminal vesicles, bladder, ureters, urethra, and bones), skeletal muscles (obturator, piriformis, elevator muscle of anus, ischiococcygeus, puborectalis, and external anal sphincter), sciatic or sacral nerves, sacrospinous/sacrotuberous ligaments, any extramesorectal vessel, any loop of colon or small intestine outside the primary lesion, and extramesorectal fiber [13].
Staging of rectal cancer extending into the anal canal requires detailed assessment of the anal canal and sphincter complex using high-resolution T2-WI in the coronal plane parallel to the anal canal. The “cT” category is recommended to be determined primarily based on the extent of the tumor at the rectal level. Involvement of the external sphincter, puborectalis, and elevator muscle of anus should be classified as cT4b. Extension into the anal canal should be described separately, detailing the affected structures (internal sphincter, intersphincteric space, and/or pelvic floor). The report should additionally indicate whether the is positive (+) or negative (−) [13]. For tumors that have grown into the anal canal below the dentate line, the inguinal lymph nodes may be considered regional (as defined in AJCC-TNM8).
Locally advanced rectal cancer is a primary tumor that has grown beyond the muscular layer (T3/T4) and/or affects regional lymph nodes (N1/2) but has no signs of distant metastases (M0).
Circumferential resection margin (CRM) is a surgery and pathology term defined as the surface of surgical excision of the non-peritoneal part of the rectum, which should pass along the mesorectal fascia when performing a total mesorectumectomy.
The status of CRM is determined by histological examination of the surgically removed rectum specimens. It can be predicted based on MRI by the shortest distance between the extramural component of the tumor/deposit/affected lymph node and the mesorectal fascia. Involvement of the CRM is indicated as CRM(+) if this distance is ≤1 mm. For low-lying rectal cancer, the shortest distance is determined to the elevator muscle of anus. The distance from enlarged lymph nodes without signs of extracapsular spread (with smooth contours) is not considered and should be regarded as CRM(−) [13].
Extramural vascular/venous invasion in histological examination represents intravascular growth of the tumor beyond the rectal wall, serving as a predictor of poor prognosis, lymphatic and distant metastases, and tumor recurrence [14–17]. On T2-WI, extramural vascular/venous invasion is characterized by the spread of a tumor signal into the vascular structures of the mesorectal tissue [6, 18, 19], which can be combined with the increased diameter of the affected vessel or with the tumor extending beyond its walls with the formation of a node, beaded, or worm-like structure. It is important to note that the MRI assessment of extramural venous invasion in vessels less than 3 mm in diameter is unreliable. When determining the category “T” (T3 and T4 tumors) in cases of fusion of the primary tumor and an extramural venous invasion lesion, their total size should be considered.
Tumor adhesion is considered in imaging when it is challenging to clearly trace the fatty tissue between the tumor and the neighboring organ. In this situation, there is no MR signal from the tumor tissue within the structure of the adjacent neighboring organ. This option is recommended to be regarded as “possible invasion” [20]. According to the AJCC-TNM8, it should be classified as “mrT4b,” with subsequent clarification of the stage after surgery. If microscopic examination does not reveal tumor elements at the adhesion site, then such a case is classified as “pT1-3,” depending on the depth of invasion.
Terms related to describing colorectal cancer
The description of a tumor does not affect staging, but it is important for characterizing the tumor. Here are terms used to describe tumors.
Desmoplastic reaction is a fibrous reaction of the tumor stroma that occurs at the border with normal tissue in the form of connective tissue spicules without tumor cells. It can be observed both in the primary tumor, making it difficult to differentiate T2 and T3a-b tumors on MRI and after neoadjuvant chemoradiotherapy.
Mucinous cancer is a prognostically unfavorable histological variant of rectal cancer with tumor content of extracellular mucin >50% of the tumor volume. On MRI, mucin accumulations have a hyperintense signal on T2-WI (Fig. 3).
Fig. 3. Variants of tumor image on T2-WI. (a) Polypoid/exophytic tumor (arrow). (b) Semicircular tumor (T), extramural vascular invasion (arrows). (c) Mucinous tumor (arrows).
The tumor lesion is usually represented by ulceration/erosion in the center of the tumor, where the maximum depth of tumor invasion is determined.
Polypoid tumor is a tumor with an exophytic type of growth (Fig. 3). It may have a pedicle with clearly visible feeding vessels. The location of such a tumor can be indicated using a conventional dial (12 o’clock for the center of the anterior wall, 6 o’clock for the center of the posterior wall, 3 o’clock for the center of the left wall, and 9 o’clock for the center of the right wall).
A semicircular tumor occupies only part of the circumference of the rectum.
Circular/subcircular tumor spreads over the entire or almost entire circumference of the rectal lumen (Fig. 3).
Terms related to localization, staging, and criteria for lymph node involvement in rectal cancer
Lymph node assessment is an important aspect of rectal cancer staging, although it is less precise than for T category [21]. According to a meta-analysis [22], the sensitivity and specificity of MRI in the assessment of lymph node involvement are 73% (95% CI 68–77) and 74% (95% CI 68–80), respectively. Computed tomography and transrectal ultrasound demonstrate diagnostic effectiveness comparable with MRI [23].
Not all lymph nodes located in the pelvis are regional for rectal cancer and are classified as “N.” When assessing pelvic lymph nodes as regional, it is important to consider their location (Fig. 4) and, if possible, indicate it in the examination report. Here are terms related to location, staging, and criteria for lymph node involvement.
Category “N” according to the TNM system: N0: absence of abnormal locoregional lymph nodes; N1: 1 to 3 abnormal regional lymph nodes (N1a: 1 lymph node; N1b: 2-3 lymph nodes; N1c: tumor deposit); N2: >3 abnormal regional lymph nodes (N2a: 4 to 6 lymph nodes; N2b: ≥7 lymph nodes). When describing, it is acceptable to use “N(+)” in the presence of abnormal regional lymph nodes, regardless of their number, and “N(−)” in the absence of abnormal regional lymph nodes.
Regional lymph nodes (N). According to the latest AJCC-TNM8, regional lymph nodes include mesorectal/pararectal, superior rectal, inferior mesenteric, and internal iliac lymph nodes, without specific mention of the obturator lymph nodes. However, the obturator lymph nodes are usually classified as regional lymph nodes [21]. All other pelvic lymph nodes, including inguinal, external iliac, common iliac, and para-aortic lymph nodes, are not regional in rectal cancer and should be regarded as distant metastases (M).
Mesorectal lymph nodes are located in the mesorectum and are regional ones.
The lower mesenteric and upper rectal lymph nodes are classified as regional and are removed during a total mesorectumectomy. It is recommended to mark the most suspicious upper lymph node of these chains, as it may change the site of vessel ligation during total mesorectumectomy.
Morphological criteria for involvement of mesorectal, superior rectal, and inferior mesenteric lymph nodes include (a) uneven boarders, (b) heterogeneous structure, and (c) rounded shape. Suspicious lymph nodes are (a) <5 mm in size if three morphological criteria are met; (b) 5 to 9 mm in size if two criteria are met; and (c) >9 mm in all cases. After neoadjuvant chemoradiotherapy, all lymph nodes larger than 5 mm should be considered suspicious [8].
A deposit is defined in pathology as a single tumor nodule in the mesorectal tissue without evidence of identifiable lymph node tissue or vascular/nervous structures. It is designated as “N1c,” regardless of the number of deposits. The number of tumor deposits is not added to the number of positive lymph nodes [24]. To date, there is insufficient evidence regarding MRI’s ability to reliably differentiate between lymph nodes and tumor deposits [13]. Deposits may form due to intermittent tumor spread, lymphatic spread, venous or perineural invasion, or complete lymph node replacement [25]. On MRI, distinguishing between positive lymph nodes with extracapsular extension, extranodal tumor deposits, and intermittent extramural vascular invasion is challenging. Available evidence suggests that all these conditions have a worse prognosis than lymph node involvement [26]. Tumor deposits in the mesorectum or along the large rectal vessels, combined with signs of extramural vascular/venous invasion, are regarded as “N1c,” EMVI(+). Deposits without signs of extramural vascular/venous invasion are regarded as “N1c,” EMVI(−).
The lateral pelvic lymph nodes, situated at the side pelvic walls, are lymph nodes external to the mesorectal fascia, including the external, internal iliac, and obturator lymph nodes. When describing them, it is better, whenever possible, to indicate a more specific location. During primary staging, it is recommended to consider regional lateral pelvic lymph nodes (internal iliac and obturator) with a short axis (≥7 mm) as suspicious [12, 27]. Morphological criteria for lateral pelvic lymph nodes are not recommended [13].
The internal iliac lymph nodes are regional and, in the case of rectal cancer, are included in the scope of dissection of the lateral pelvic lymph nodes. They are located along the internal iliac vessels. At the level of the obturator muscle, they are localized medially to the internal iliac artery; lymph nodes lateral to the internal iliac artery are considered obturator lymph nodes (Fig. 4).
Fig. 4. Localization of the lateral pelvic lymph nodes (colored): external iliac lymph nodes are red; obturator lymph nodes are blue; and internal iliac lymph nodes are green. Shown in levels (a) proximal and (b) distal. EIA: external iliac artery; EIV: external iliac vein; IIV: internal iliac vein; IIA: internal iliac artery; Obt a/v/n: obturator artery/vein/nerve; OIM: obturator internus muscle.
The obturator lymph nodes are regional. They are located between the external and internal iliac arteries, medially to the internal obturator muscle and laterally to the internal iliac artery (Fig. 4).
The external iliac lymph nodes are not regional. They are located along the external iliac vessels (Fig. 4) and are divided into lateral, middle/median, and medial chains. The lateral subgroup is located laterally to the external iliac artery. The middle/median group is between the artery and the vein. The medial group is posterior to the external iliac vein. The lymph nodes in the medial subgroup are located near the obturator vessels and obturator lymph nodes. This can cause diagnostic difficulties because they are often indistinguishable from the obturator lymph nodes localized along the obturator artery at the point of its origin from the internal iliac (hypogastric) artery at the level of the internal obturator muscles [3]. Involvement of the external iliac lymph nodes in rectal cancer is extremely rare. Non-regional lymph nodes are considered suspicious if they measure >10 mm in short axis.
Inguinal lymph nodes are not regional in rectal cancer, but are regional in squamous cell carcinoma of the anal canal. They are located in the groin area below the inguinal ligament. They can be classified as regional for tumors extending below the dentate line [13]. They are divided into superficial (anterior to the saphenous vein and superficial femoral vessels) and deep (medial to the femoral vessels).
Terms to describe response to neoadjuvant chemoradiation therapy
Currently, a combination neoadjuvant chemoradiation therapy is widely used to treat patients with rectal cancer, significantly affecting the planning of further treatment. MRI is currently considered the optimal imaging modality for assessing effectiveness of neoadjuvant chemoradiation therapy. Here are terms recommended to describe response to neoadjuvant chemoradiation therapy.
Pathologic complete response (pCR) is a response to neoadjuvant chemoradiation therapy, characterized by the complete absence of viable tumor cells during pathological examination of the surgical specimen.
Clinical complete response (cCR) is a response to neoadjuvant chemoradiotherapy, characterized by the absence of a clinically detectable tumor during digital rectal examination, MRI, and endoscopy. It is used as a surrogate for pCR. On MRI, it represents either subtle fibrosis of the rectal wall in the tumor bed without residual areas of tumor signal or recovery of a normal rectal wall without any evidence of tumor [28].
Near-complete response was introduced because some patients, initially showing a good but incomplete response during the follow-up examination, may be re-evaluated after a longer interval after neoadjuvant chemoradiation therapy and may achieve a cCR.
Downstaging is a term describing downstaging of the T or N category after neoadjuvant chemoradiation therapy. The post-treatment category is indicated by the prefix “y,” for example, yT0 means no visible primary tumor.
Downsizing refers to the decrease in size of a tumor or its regional metastases after neoadjuvant therapy without changing T or N categories.
Tumor regression grading (TRG) is a system for assessing the response to neoadjuvant chemoradiotherapy, derived from a modification of the Mandard pathological staging (MRI TRG). It involves a qualitative assessment of the ratio of a low MR signal from fibrous tissue and a medium-intensity signal from a residual tumor on T2-WI.
- mrTRG1 (complete response) means no macroscopic signs of residual tumor tissue/a minimal area of fibrosis is visualized (thin scar).
- mrTRG2 (significant/almost complete response) means the presence of dense fibrous scar, with no signs of tumor tissue visualized (according to pathological data, tumor cells are absent/single in settings of dense fibrosis).
- mrTRG3 (moderate response) means fibrosis predominates (>50%), while an MR signal of medium intensity is visualized, which is characteristic for tumor tissue.
- mrTRG4 (minimal response) means the MR signal from the tumor tissue predominates, combined with a small/minimal structural fibrosis.
- mrTRG5 (no response/progression) means only an MR signal of medium intensity, characteristic of tumor tissue, without signs of fibrosis (Fig. 5).
Fig. 5. Assessment of tumor regression on high-resolution T2-WI using TRG. TRG١: (a) Tumor located at the 12–2 o’clock position before neoadjuvant chemoradiation therapy (arrow); (b) after treatment, the tumor is replaced by a linear area of submucosal fibrosis. TRG2: (c) Tumor in the lower ampullary rectum before chemoradiation therapy (arrow); (d) after treatment, the tumor is determined as an area of thick fibrosis (arrow), without macroscopic MR signs of tumor. TRG3: (e) Semicircular tumor in the lower ampullary rectum before chemoradiation therapy (arrow); (f) after treatment, the tumor has a mixed MR signal with a predominance of a low-intensity signal, characteristic of fibrosis, and preservation of macroscopic areas of a tumor MR signal of medium intensity (arrow). TRG4: (g) Tumor before chemoradiation therapy (arrow); (h) after treatment (arrow), there are no signs of response to treatment; the MR signal of the tumor tissue persists.
Current clinical experience shows that this system has poor correlation with pathological TRG, limited positive predictive value of pCR and poor reproducibility with low kappa values [29, 30]. Therefore, further research is required to find ways of improving its diagnostic efficiency.
The scar in the irradiated tumor bed is characterized by a hypointense T2-WI signal without signs of diffusion limitation on diffusion-weighted images.
Submucosal edema is identified after neoadjuvant chemoradiation therapy as an area of high signal intensity on T2-WI in the rectal wall adjacent to the treated tumor and should not be misinterpreted as a tumor.
Mucinous/colloid degeneration (mucinous response) is characterized by high T2-WI signal of acellular mucin accumulations that lack viable tumor cells. It may be observed in non-mucinous tumors after neoadjuvant chemoradiation therapy. In the case of a mucinous tumor, remaining post-treatment mucin may also not contain tumor cells. However, it is difficult to distinguish between acellular and cellular mucin using MRI.
Terms to describe treatment options for colorectal cancer
Surgery is the main treatment option for rectal cancer. In the case of locally advanced tumor, it is used after neoadjuvant treatment. Here are terms used to describe treatment options for colorectal cancer.
A total mesorectumectomy involves excision of the rectum along the mesorectal fascia en bloc with mesorectal fat, vessels, and lymph nodes. It is considered to be the gold standard for radical treatment of colorectal cancer [31].
Partial mesorectumectomy involves partial removal of mesorectal tissue, followed by its intersection and preservation of part of the mesorectal tissue of the anastomosed area of the rectum.
Anterior resection/low anterior resection is the most common type of surgery in rectal cancer, accompanied by a total or partial mesorectumectomy and formation of a colorectal anastomosis.
Intersphincteric resection is a sphincter-sparing operation for low-grade rectal cancer, with only part of the internal anal sphincter removed and with the external anal sphincter preserved. This is followed by a coloanal anastomosis. It can be used in some cases when the intersphincteric space is not infiltrated by a tumor.
Abdominoperineal excision involves resection of the entire sphincter complex with formation of a permanent colostomy.
Abdominal-anal resection of the rectum is a treatment method involving a total mesorectumectomy but with formation of a coloanal anastomosis.
Extralevator abdominoperineal excision is a variant of the standard abdominoperineal excision with a wider excision of the sphincter complex and the elevator muscle of anus.
Pelvic exenteration involves radical resection en bloc of all pelvic organs affected by the tumor, often followed by visceral reconstruction, including restoration of the passage for intestinal contents and using one of the urine diversion methods [32]. The 5-year overall survival rate after pelvic exenteration for primary locally advanced rectal cancer is 30–55%; for recurrent tumors, it usually does not exceed 20–25% [33].
Transanal excision is local excision of the tumor through its entire thickness to the mesorectal tissue. Lymph nodes are not removed.
Transanal endoscopic microsurgery is excision of the tumor to the full thickness of the mesorectal tissue using video endoscopic technologies. This technique provides a higher visibility of the rectal wall and higher possibility of its full-thickness excision with subsequent suturing of the defect.
R0/R1/R2 surgeries are categorized in accordance with the accepted criteria for the radicality of operations. R0 is radical removal of the tumor without microscopic and macroscopic residual tumor. R1 is marginal resection, microscopic residual tumor. R2 is incomplete tumor removal, macroscopic residual tumor. Residual tumor should be determined after a subjective assessment by a surgeon and an objective morphological examination.
Preoperative radiation therapy is conducted as a prolonged course (5–6 weeks) in combination with radiosensitizing chemotherapy or as a short, concentrated course (1 week) without concomitant chemotherapy.
Neoadjuvant chemoradiation therapy is a combination of chemotherapy and radiation therapy, which is conducted before surgery. Chemotherapy here is considered radiosensitizing and not “systemic” (for the treatment of distant metastases).
Neoadjuvant chemotherapy is a type of chemotherapy administered immediately before surgical removal of the primary tumor to improve outcomes of surgery/radiation therapy and prevent metastases.
CONCLUSION
Based on expert consensus, a vocabulary has been prepared to provide diagnostic radiology specialists with terms for describing and interpreting results of rectal cancer imaging.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work
About the authors
Tatiana P. Berezovskaya
A.F. Tsyba Medical Radiological Research Center ― branch National Medical Research Radiological Center
Email: tberezovska@yahoo.com
ORCID iD: 0000-0002-3549-4499
SPIN-code: 5837-3465
MD, Dr. Sci. (Med.), Professor
Russian Federation, ObninskNatalia A. Rubtsova
P.A. Herzen Moscow Research Oncological Institute ― branch National Medical Research Radiological Center
Email: rna17@ya.ru
ORCID iD: 0000-0001-8378-4338
SPIN-code: 9712-9091
MD, Dr. Sci. (Med.)
Russian Federation, MoscowValentin E. Sinitsyn
Lomonosov Moscow State University
Email: vsin@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-code: 8449-6590
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowIrina V. Zarodnyuk
State Scientific Centre of Coloproctology
Email: zarodnyuk_iv@gnck.ru
ORCID iD: 0000-0002-9442-7480
SPIN-code: 8310-8989
MD, Dr. Sci. (Med.)
Russian Federation, MoscowNicolai V. Nudnov
Russian Scientific Center of Roentgenoradiology
Email: nudnov@mrrc.ru
ORCID iD: 0000-0001-5994-0468
SPIN-code: 3018-2527
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowAndrei V. Mishchenko
N.N. Petrov National Medical Research Centre of Oncology
Email: dr.mishchenko@mail.ru
ORCID iD: 0000-0001-7921-3487
SPIN-code: 8825-4704
MD, Dr. Sci. (Med.)
Russian Federation, MoscowYuliya L. Trubacheva
State Scientific Centre of Coloproctology
Email: trubacheva_ul@gnck.ru
ORCID iD: 0000-0002-8403-195X
SPIN-code: 3427-9074
MD, Dr. Sci. (Med.)
Russian Federation, MoscowTatiana A. Bergen
E. Meshalkin National Medical Research Center
Email: tbergen@yandex.ru
ORCID iD: 0000-0003-1530-1327
SPIN-code: 5467-7347
MD, Dr. Sci. (Med.)
Russian Federation, MoscowPavel Yu. Grishko
N.N. Petrov National Medical Research Centre of Oncology
Email: dr.grishko@mail.ru
ORCID iD: 0000-0003-4665-6999
SPIN-code: 3109-1583
MD, Cand. Sci. (Med.)
Russian Federation, MoscowSvetlana S. Balyasnikova
N.N. Blokhin National Medical Research Center of Oncology
Email: Balyasnikova.Svetlana@gmail.com
ORCID iD: 0000-0002-9666-9301
SPIN-code: 3987-2336
MD, Cand. Sci. (Med.)
Russian Federation, MoscowYana A. Dayneko
A.F. Tsyba Medical Radiological Research Center ― branch National Medical Research Radiological Center
Email: vorobeyana@gmail.com
ORCID iD: 0000-0002-4524-0839
MD, Cand. Sci. (Med.)
Russian Federation, ObninskDarya V. Ryjkova
Almazov National Medical Research Centre
Email: d_ryjkova@mail.ru
ORCID iD: 0000-0002-7086-9153
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowMalika M. Hodzhibekova
P.A. Herzen Moscow Research Oncological Institute ― branch National Medical Research Radiological Center
Email: malika_25@mail.ru
ORCID iD: 0000-0002-2172-5778
SPIN-code: 3999-7304
MD, Dr. Sci. (Med.)
Russian Federation, MoscowNataliya A. Rucheva
V.I. Shumakov National Medical Research Center of Transplantology and Artificial Organs
Email: rna1969@yandex.ru
ORCID iD: 0000-0002-8063-4462
SPIN-code: 2196-8300
MD, Cand. Sci. (Med.)
Russian Federation, MoscowIgor E. Turin
N.N. Blokhin National Medical Research Center of Oncology
Email: igortyurin@gmail.com
ORCID iD: 0000-0002-8587-4422
SPIN-code: 6499-2398
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowSergey I. Achkasov
State Scientific Centre of Coloproctology
Email: achkasovy@mail.ru
ORCID iD: 0000-0001-9294-5447
SPIN-code: 5467-1062
MD, Dr. Sci. (Med.), Professor, Corresponding Member of the Academy of Sciences
Russian Federation, MoscowAlexey A. Nevolskikh
A.F. Tsyba Medical Radiological Research Center ― branch National Medical Research Radiological Center
Email: alexey.nevol@gmail.com
ORCID iD: 0000-0001-5961-2958
SPIN-code: 3787-6139
MD, Dr. Sci. (Med.)
Russian Federation, ObninskSergey S. Gordeev
N.N. Blokhin National Medical Research Center of Oncology
Email: ss.netoncology@gmail.com
ORCID iD: 0000-0002-9303-8379
SPIN-code: 6577-5540
MD, Dr. Sci. (Med.)
Russian Federation, MoscowInna V. Droshneva
P.A. Herzen Moscow Research Oncological Institute ― branch National Medical Research Radiological Center
Author for correspondence.
Email: droshnevainna@mail.ru
SPIN-code: 1908-2624
MD, Cand. Sci. (Med.)
Russian Federation, MoscowReferences
- Rectal cancer. Clinical recommendations. Approved at the meeting of the Scientific and Practical Council of the Ministry of Health of the Russian Federation. Moscow; 2022.(In Russ). Available from: https://cr.minzdrav.gov.ru/recomend/554_3. Accessed: 15.08.2023.
- Bogveradze N, Snaebjornsson P, Grotenhuis BA, et al. MRI anatomy of the rectum: Key concepts important for rectal cancer staging and treatment planning. Insights Imaging. 2023;14(1):13. doi: 10.1186/s13244-022-01348-8
- Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol. 2018;43(11):2893–2902. doi: 10.1007/s00261-018-1642-9
- Nougaret S, Rousset P, Gormly K, et al. Structured and shared MRI staging lexicon and report of rectal cancer: A consensus proposal by the French Radiology Group (GRERCAR) and Surgical Group (GRECCAR) for rectal cancer. Diagn Interv Imaging. 2022;103(3):127–141. doi: 10.1016/j.diii.2021.08.003
- Grishko PY, Balyasnikova SS, Samsonov DV, et al. A modern view on the principles of diagnosis and treatment of rectal cancer according to MRI data (literature review). Medical Visualization. 2019;23(2):7–26.(In Russ). doi: 10.24835/1607-0763-2019-2-7-26
- Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol. 2022;(43):101739. doi: 10.1016/j.suronc.2022.101739
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):22–40. doi: 10.1093/anonc/mdx22 4
- Beets-Tan R, Lambregts D, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–1475. doi: 10.1007/s0033 0-017-5026-2
- Oien K, Forsmo HM, Rösler C, et al. Endorectal ultrasound and magnetic resonance imaging for staging of early rectal cancers: How well does it work in practice? Acta Oncol. 2019;58(Sup1):49–54. doi: 10.1080/0284186X.2019.1569259
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Wiley-Blackwell; 2017. 272 р.
- Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38(12):1286–1295. doi: 10.1007/BF02049154
- Boot J, Gomez-Munoz F, Beets-Tan R. Imaging of rectal cancer. Radiologe. 2019;59(Suppl 1):46–50٠. doi: 10.1007/s00117-019-0579-5
- Lambregts D, Bogveradze N, Blomqvist L, et al. Current controversies in TNM for the radiological staging of rectal cancer and how to deal with them: Results of a global online survey and multidisciplinary expert consensus. Eur Radiol. 2022;32(7):4991–5003. doi: 10.1007/s00330-022-08591-z
- Mainovskaya OA, Rybakov EG, Chernyshov SV, et al. New morphological risk factors for metastasis to regional lymph nodes in rectal cancer with invasion of the submucosal base. Coloproctology. 2021;20(4):22–33. (In Russ). doi: 10.33878/2073-7556-2021-20-4-22-33
- Volkova SN, Stashuk GA, Chermensky GV, Naumov EK. The role of MRI in the detection of extramural vascular invasion as an indicator of the presence of regional and distant metastases of cancer of the lower ampullary rectum. Experimental Clin Gastroenterol. 2019;164(4):66–71. (In Russ). doi: 10.31146/1682-8658-ecg-164-4-66-71
- Lord AC, D’Souza N, Shaw A, et al. MRI-diagnosed tumor deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg. 2022;276(2):334–344. doi: 10.1097/SLA.0000000000004499
- Rokan Z, Simillis C, Kontovounisios C, et al. Locally recurrent rectal cancer according to a standardized MRI classification system: A systematic review of the literature. J Clin Med. 2022;11(12):3511. doi: 10.3390/jcm11123511
- Grishko PY, Mishchenko AV, Ivko OV, et al. The possibilities of multiparametric magnetic resonance imaging in assessing the effectiveness of neoadjuvant treatment of rectal cancer. Radiation Diagnostics Therapy. 2019;10(4):49–56.(In Russ).
- Inoue A, Sheedy SP, Heiken JP, et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging. 2021;(2):110. doi: 10.1186/s13244-021-01023-4
- Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic Accuracy of MRI for assessment of t category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta-analysis. Ann Sur Oncol. 2012;19(7):2212–2222. doi: 10.1245/s10434-011-2210-5
- Borgheresi A, De Muzio F, Agostini A, et al. Lymph nodes evaluation in rectal cancer: Where do we stand and future perspective. J Clin Med. 2022;11(9):2599. doi: 10.3390/jcm11092599
- Zhuang Z, Zhang Y, Wei M, et al. Magnetic resonance imaging evaluation of the accuracy of various lymph node staging criteria in rectal cancer: A systematic review and meta-analysis. Front Oncol. 2021;(11):709070. doi: 10.3389/fonc.2021.709070
- Li X, Sun Y, Tang L, et al. Evaluating local lymph node metastasis with magnetic resonance imaging, endoluminal ultrasound and computed tomography in rectal cancer: A meta-analysis. Color Dis. 2015;17(6):129–135. doi: 10.1111/codi.12909
- Weiser MR. AJCC 8th ed. Colorectal cancer. Ann Surg Oncol. 2018;25(6):1454–1455. doi: 10.1245/s10434-018-6462-1
- Ueno H, Nagtegaal ID, Quirke P, et al. Tumor deposits in colorectal cancer: Refining their definition in the TNM system. A G Surg. 2023;7(2):225–235. doi: 10.1002/ags3.12652
- Santiago I, Figueiredo N, Parés O, et al. MRI of rectal cancer: Relevant anatomy and staging key points. Insights Imaging. 2020;11(1):100. doi: 10.1186/s13244-020-00890-7
- Ogura A, Konishi T, Cunningham C, et al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: Results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. 2019;37(1):33–43. doi: 10.1200/JCO.18.00032
- Gollub MJ, Costello JR, Ernst RD, et al. A primer on rectal MRI in patients on watch-and-wait treatment for rectal cancer. Abdom Radiol. 2023. doi: 10.1007/s00261-023-03900-6
- Berezovskaya TP, Daineko YA, Nevolskikh AA, et al. Prospective evaluation of the use of the MRTG system in determining the effectiveness of neoadjuvant chemoradiotherapy in patients with rectal cancer. Bulletin Radiol Radiol. 2021;102(1):6–17. (In Russ). doi: 10.20862/0042-4676-2021-102-1-6-17
- Almeida RR, Souza D, Matalon SA, et al. Rectal MRI after neoadjuvant chemoradiation therapy: A pictorial guide to interpretation. Abdom Radiol. 2021;46(7):3044–3057. doi: 10.1007/s00261-021-03007-w
- Shelygin YA, Chernyshov SV, Kazieva LY, et al. Comparative analysis of open and transanal total mesorectumectomy in rectal cancer. Coloproctology. 2018;(4):67–73. (In Russ).
- Maistrenko NA, Khvatov AA, Sazonov AA. Pelvic exenterations in the treatment of locally advanced tumors. Bulletin Surnamed after Grekov. 2014;173(6):37–43. (In Russ).
- Sidorov DV, Alekseev BY, Grishin NA, et al. Variants of pelvic exenteration in locally advanced primary and recurrent rectal cancer. Oncology J named after P.A. Herzen. 2013;(6):7–13. (In Russ).
Supplementary files