Prospective evaluation of the extensibility of the ascending aorta wall and its vascular prosthesis in a patient with an aneurysm with technically flawless surgical correction and postoperative decrease in functional parameters: A case report
- Authors: Friedman A.V.1, Bergen T.A.1, Sirota D.A.1, Kozlov B.N.2, Zhuravleva I.Y.1, Tarkova A.R.1, Ussov W.Y.1, Chernyavskiy A.M.1
-
Affiliations:
- E. Meshalkin National Medical Research Center
- Cardiology Research Institute of the Tomsk National Research Medical Center
- Issue: Vol 5, No 2 (2024)
- Pages: 342-353
- Section: Case reports
- Submitted: 06.08.2023
- Accepted: 06.12.2023
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/568070
- DOI: https://doi.org/10.17816/DD568070
- ID: 568070
Cite item
Abstract
In this clinical case, a patient who had an instrumentally detected aneurysm with the lumen expanding up to 60 mm underwent a surgically flawless prosthetic replacement of the ascending aorta. This treatment led to decreased exercise tolerance, decreased contractile function of the left ventricular myocardium at rest, and enlarged pulmonary artery. The leading factor was a decrease in the volume of systolic expansion of the aorta down to 5 mL (at the initial 13 mL), despite a noticeable increase in the extensibility and a decrease in mechanical stiffness compared with initial indexes of the affected aortic wall. In the literature review, considering mechanical extensibility and elasticity, problems in creating aortic prostheses equivalent to those for healthy biological tissues were discussed.
Full Text
INTRODUCTION
Surgical treatment of ascending aortic aneurysms with a >5 cm lumen expansion [1] has been the technique of choice in preventing the risk of aneurysm rupture, with a mortality rate of up to 100% in acute cases [2–4].
Several cardiac surgery techniques, involving complete or partial arch replacement, have been developed [5].
The outcomes of such replacement are assessed based on the significant decrease in the risk of mortality in such patients [2]. The quantitative assessment of physiological and biomechanical parameters of the ascending aorta, quality of life, and the presence and severity of angina and other coronary ischemia markers is often regarded as less significant [3]. This may be justified, as saving the patient’s life is always the top concern.
However, a sustained decrease in the mortality rate with the surgical treatment of ascending aortic aneurysm requires further clarification of pathophysiological criteria determining the functional status of patients, possibility of recovery and vocational rehabilitation, state of the cardiac muscle, and factors affecting coronary blood supply.
The ascending aorta is a crucial anatomical and functional component of the vascular system. It provides blood supply to the cardiac muscle, which invariably occurs during diastole, within the systolic expansion volume of the ascending aorta [6]. Physiologists and sports medicine specialists [6], as well as prosthetic valve and vessel manufacturers [7], have long been interested in the elastic properties of the ascending aorta in the context of adequate blood supply to the cardiac muscle, when the aorta, which stretches during systole, collapses during diastole. However, single-center and multicenter studies of the clinical use of biophysical and biomechanical assessments of aortic elasticity are limited [4]. It has been previously demonstrated that decreased elasticity and distensibility and increased stiffness of the ascending aorta are significant pathological factors contributing to the risk of acute myocardial infarction [8, 9]. Studies of ascending aortic elasticity in cardiac surgery patients with aneurysms are equally clinically significant. Insufficient graft elasticity can be a limiting and pathological factor.
Thus, this study presents a clinical case of a female patient who failed to reach the target pO2 level post-surgery, as well as other parameters required for a good performance status (e.g., exercise tolerance), despite uncomplicated ascending aortic replacement. These metrics did not improve, but rather worsened, and the patient has been dependent on an oxygen concentrator for breathing several months after surgery.
DESCRIPTION OF THE CASE
We present a clinical case of prospective follow-up of changes in biomechanical parameters of aortic aneurysm during surgical treatment of a 65-year-old female (patient B-k). The patient has a history of hypertension for 10 years (with full pharmacological blood pressure control). Additionally, she has type 2 diabetes mellitus, with glucose and glycated hemoglobin levels controlled to the limit of normal with oral antidiabetic drugs. She was otherwise healthy for the last 15 years.
The patient initially presented to a neurologist with increasingly frequent episodes of dizziness and weakness with fatigue, and transient, short-term episodes of loss of speech. Critical stenosis of the internal carotid artery or its branches was suspected, and the patient was referred for ultrasound examination and carotid magnetic resonance (MR) angiography. These examinations did not confirm the carotid artery pathology. Narrowing of the internal carotid arteries or their branches by more than 15%–20% was not observed. However, carotid MR angiography showed a pathological radial expansion of the ascending aorta lumen of up to 57–60 mm. This was confirmed by MR aortography (Fig. 2a), and the patient was referred to the Research Institute of Cardiology of the Tomsk National Medical Research Center for consultation and cardiac surgery.
Further, the patient had preoperative coronary angiography and aortography, which confirmed the nature and extent of the aortic lesion and ruled out coronary stenosis. The proximal right coronary artery showed the most severe stenosis, of up to 35% of the lumen. Stenoses in the left coronary artery did not exceed 25% in any of its branches.
Prior to aortic replacement, the exercise tolerance threshold according to a cycle ergometer exercise test with electrocardiography (ECG) monitoring was 25 W. The test was stopped because of shortness of breath and muscular weakness. The test did not reveal ECG signs of coronary insufficiency.
As previously mentioned, the patient had magnetic resonance imaging (MRI) of the heart and aortic wall with ECG gating [8], including the thoracic aorta up to the diaphragm. In particular, heart MRI along its short and long axes was performed, which included the following:
- T1 weighted images (WI): time of repetition (TR), 500 msec; time of echo (TE), 12 msec
- T2WI: TR, 4,000 msec; TE, 25 msec
- Steady-state free precession (SSFP) images
Slice thickness: 5–8 mm; matrix: 256 × 392 or 256 × 256. Axial T1WI chest MRI with respiration and ECG gating, with increased TR of 1,850–1,900 ms and TE of 32 ms, was performed as a component of cardiac and chest MRI with ECG gating (Fig. 1). This mode provides visualization of large thoracic vessels, including their walls. Owing to the borderline glomerular filtration rate (<30 mL/min × 1.73 m2), additional paramagnetic contrast enhancement was not used.
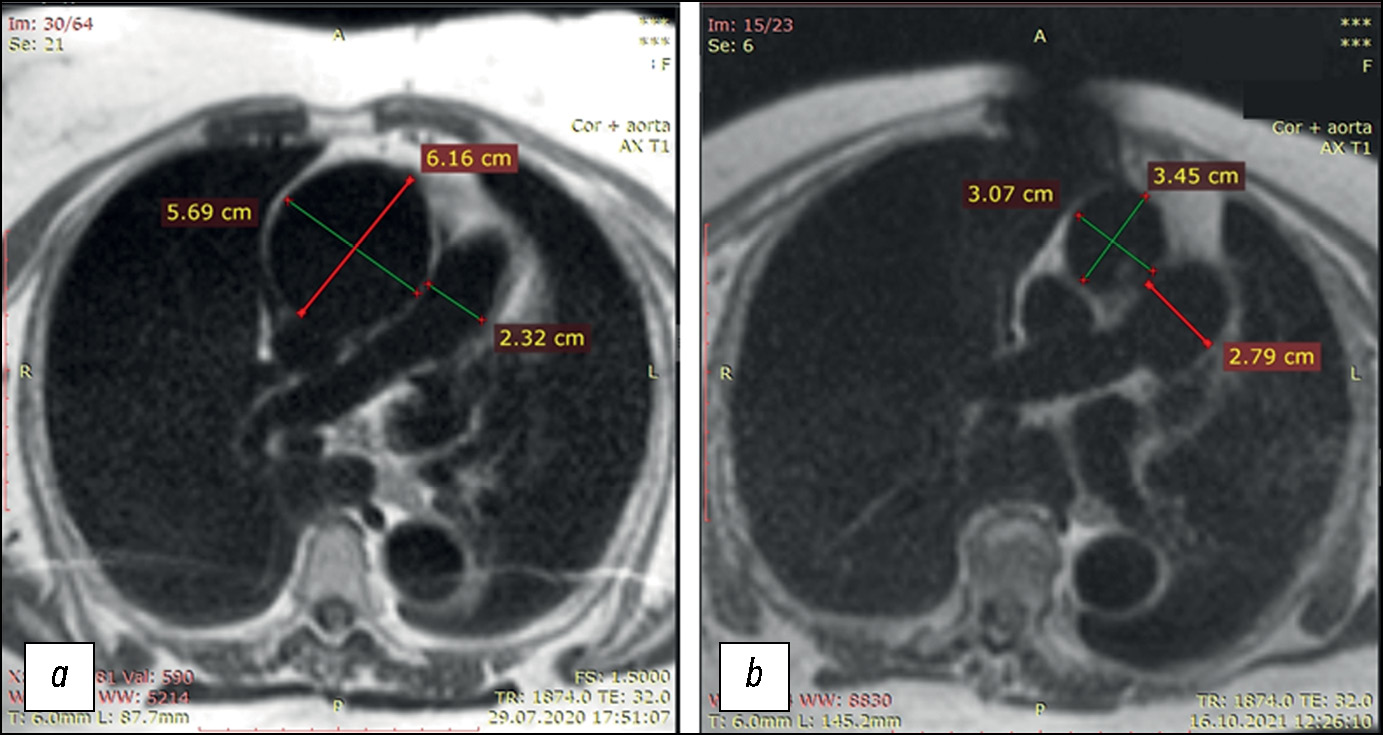
Fig.1. Transverse slices of T1-weighted images of the chest organs, including the thoracic aorta, at the pulmonary artery bifurcation level in patient B-k: (a) before prosthetic replacement of the thoracic aorta expanded due to aneurysm; critical ascending aortic aneurysm with a >6 cm lumen expansion; (b) after prosthetic replacement of the thoracic aorta expanded due to aneurysm; normal lumen of the ascending aorta. The descending aorta was normal before and after surgery. The pulmonary artery expanded to 27 mm after surgery, compared to 23 mm at admission. Postoperative tomography revealed an artifact in the chest area due to a wire fixator.
Following cardiac MRI, the patient underwent MRI of the ascending aorta with ECG gating, at the level of crossover with the pulmonary artery bifurcation level, in axial plane, in the cine mode (24 cine frames per cardiac cycle), with the assessment of changes in the aortic wall thickness during a cardiac cycle (Fig. 2b) and diameter and cross-sectional area of the lumen at the study level (marked with an arrow in Fig. 2a). The cardiac MRI findings were processed using a standard method; the left ventricular end-diastolic volume, left ventricular end-systolic volume, and left ventricular ejection fraction were calculated. Moreover, biomechanical parameters of aortic distensibility were obtained based on non-contrast-enhanced cine mode MRI findings.
Fig. 2. Magnetic resonance imaging with ECG gating in patient B-k: (a) magnetic resonance angiography of the thoracic aorta. The lumen at the supravalvular and aortic arch levels and the distances between them, which are used to calculate the ascending aorta volume during systole and diastole and the systolic expansion volume, are shown. The turquoise horizontal line with arrows at the ends represents the tomographic slice level; (b) transverse tomographic slice of the ascending aorta in the wall area, with thickness measurements for the subsequent calculations of Young’s modulus parameters. The measurements are marked by thin green lines, with respective values.
These measurements and a linear biophysical model [10, 11] were used to calculate the distensibility (radial expansion) of the aorta [12]:
Distensibilityadj = Ssyst – Sdiast / Sdiast (1)
Moreover, the distensibility adjusted for pulse pressure was calculated:
(2),
where Ssyst and Sdiast are the cross-sectional areas of the aorta during systole and diastole, respectively, and BPpulse is the pulse pressure (Fig. 3).
Fig. 3. Cross-sectional dimensions and areas of the ascending aorta during systole and diastole: top row: baseline (at admission; before replacement of the aorta that expanded due to aneurysm); bottom row: after replacement with a synthetic graft; (a, c) diastole; (b, d) systole. Of note is a considerable lumen narrowing after surgery, with a relatively small distensibility of the ascending aorta.
The transverse Young’s modulus for the ascending aorta wall was calculated based on the findings of MR aortography with ECG gating, according to the method well-studied in biomechanical experiments [10, 11]:
(3),
where E = Young’s modulus (Pa),
ddiast = transverse aortic diameter during diastole,
∆dpulse = increase in the aortic diameter during systole,
0.25 = squared Poisson’s ratio for the aortic wall, which is known to be 0.5 [11],
h = aortic wall thickness during diastole (Fig. 2b),
BPpulse = pulse pressure, and
133.3 = conversion factor (mmHg to Pa).
The ascending aortic volume was calculated, from the supravalvular level to the middle of the aortic arch (between the brachiocephalic trunk and opening of the left common carotid artery), during systole and diastole. The ascending aorta was visualized as a deformed, incompressibly curved, truncated cone with the length l (length of the aortic valve–middle of the aortic arch area; Fig. 1a), with the base radius determined by transverse slices in the cine mode: lower base radius, R, and upper base radius, r. In this case, the volume of the deformed truncated cone (the ascending aorta) can be with high accuracy estimated as follows [13]:
V = 1⁄3πl (R2 + Rr + r2) (4)
The systolic expansion volume of the aorta ΔVsyst was determined by the difference between systolic and diastolic volumes of the ascending aorta. This parameter determines the blood volume available for the coronary blood supply to the cardiac muscle during diastole, when the primary blood supply to the cardiac muscle occurs [6, 14].
The patient underwent replacement of the ascending aorta and partial arch replacement with assisted circulation, using a 35 mm synthetic graft GORE-TEX (W.L. Gore & Associates, USA). Aortic valve replacement was not performed, as no significant aortic valve insufficiency was noted, and the area of the effective hemodynamic lumen during systole was >2.0 cm2. The brachiocephalic trunk ostium was implanted in the respective branch of the graft; postoperatively, no blood supply disturbances in the right common carotid artery and subclavian artery territories were observed.
No postoperative surgical complications, including inflammation, and signs of vital organ blood supply disturbances were noted. Sinus tachycardia at rest was reported (82–92 bpm), which worsened significantly on mild exertion. The preoperative glomerular filtration rate was 57–65 mL/min × 1.73 m2, which was maintained after surgery. The patient required long-term oxygen support, because only with then her condition was subjectively close to normal. Imaging and clinical biochemistry studies revealed no signs of postoperative myocardial infarction. Without oxygen support using a membrane oxygen concentrator, the pO2 level was 81%–83%; when a concentrator was used, this parameter increased to 93%–95% or higher (occasionally, at rest). Perfusion single-photon computed tomography with 99mTc-labeled beads revealed no signs of thrombosis or pulmonary embolism.
Following the surgery, the exercise tolerance decreased significantly compared to baseline and remained minimal during the inpatient postoperative period and after discharge. The patient resides on the second floor and can only get there by elevator; an outpatient MRI required the use of an oxygen concentrator.
A follow-up examination (cardiac MRI and MR elastography of the aortic wall) was performed 4 months after surgery; the findings compared to baseline are presented in Tables 1 and 2.
Table 1. Cardiac magnetic resonance imaging findings in patient B-k before and after aortic replacement
Left ventricular myocardium mass, g | LVEDV, mL | LVEF, % | LVESV, g | Left atrial volume, mL | Pulmonary artery diameter, mm | |
Baseline (at admission) | 165 | 79.4 | 83 | 165 | 55.7 | 23 |
After ascending aortic replacement | 161 | 94.2 | 73 | 161 | 69.4 | 28 |
Note: LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume. Parameters indicating the progression of heart failure (increased LVEDV, decreased LVEF, increased left atrial volume, and pulmonary artery expansion by 4 mm) are in bold text.
Table 2. Magnetic resonance elastometry of the ascending aortic wall in patient B-k before and after aortic replacement
Young’s modulus for the ascending aortic wall, Pa | Ascending aortic distensibility | ΔVsyst, mL | ||
Absolute | Adjusted = absolute/pulse pressure | |||
Baseline (at admission) | 0,58×106 | 0.0043 | 0.0043/25 = 1.72×10-4 | 13.28 |
After ascending aortic replacement | 0,260×106 | 0.0161 | 0.0161/20 = 8.05×10-4 | 4.95 |
Aortic elasticity parameters improved dramatically following surgery; however, they still exceeded the normal value [8]. However, the systolic expansion volume of the ascending aorta (ΔVsyst) decreased significantly due to a decrease in the aortic diameter by 2 cm.
The postoperative Young’s modulus for the aortic wall (specifically, for the ascending aortic graft) decreased, whereas the elasticity increased. However, generally, the systolic expansion volume of the aorta decreased to approximately 5 mL (Table 2), which is insufficient for adequate coronary blood supply [8]. The physical dimensions of the graft corresponded to those specified in the documents. Thus, even in the absence of significant coronary stenoses and with an ideal surgical technique of ascending aortic replacement, insufficient distensibility of the aortic wall became a critical factor, limiting exercise tolerance after surgery and contributing to left ventricular failure, although without acute myocardial infarction.
DISCUSSION
When assessing aortic stiffness, methods initially tested in animal studies are used [15, 16, 17], such as external transmission of a high-frequency mechanical wave to the aorta, using a special MRI-compatible vibration generator, followed by an MRI recording of wave transmission along the aortic wall [15, 18, 19]. This method, adapted from solid-organ elasticity studies, is commonly used [18, 19, 20].
The high-frequency method of mechanical aortic elasticity assessment allows for the calculation of this parameter throughout the anatomical study area (along the length of the aorta) [19]. However, the aortic volume at a specific level, particularly at the level of the ascending aorta, is not considered [2]. The volume of various parts of the aorta in the case of pathologies has recently become a subject of interest [15].
In this context, the distensibility of the aortic lumen during a cardiac cycle following changes in aortic pressure is a more physiological parameter [12]. Regarding the ascending aorta, it allows for direct assessment of the blood volume available for pumping into the coronary bed during diastole [12]. In the present case, this parameter allowed determining the exact cause of the patient’s postoperative condition, which was initially attributed to undetected flaws of the surgical technique; however, the existence of these flaws was later disproved.
This demonstrates that the aorta is crucial for adequate coronary blood supply to the cardiac muscle [6, 12]. In the case of ascending aortic replacement, the graft elasticity plays a critical role [7]. It is even more relevant considering that aortic wall inflammation [24] and stiffness [25] are associated with the incidence and severity of cerebrovascular accidents. Single-center [12] and multicenter studies [8, 26] confirmed that increased aortic wall stiffness is a predictor of increased incidence of coronary disorders in patients with myocardial infarction. In patients with cardiovascular diseases that do not require cardiac surgery, drug therapy can significantly improve aortic distensibility and elasticity [27].
As previously stated, further development of ascending aortic grafts is focused on the use of synthetic and multicomponent materials with preserved elasticity, which ensure adequate diastolic blood supply to the cardiac muscle and exercise tolerance [7]. Manufacturers are aware of this issue [28, 29], which is shown in the present case: the mechanical aortic wall stiffness after surgery decreased more than twofold compared to the aorta with aneurysm before surgery, whereas the distensibility increased more than threefold (Table 2). However, considering the graft diameter, which is decreased compared to the baseline aneurysm, modern synthetic materials cannot maintain the systolic expansion volume of the ascending aorta (ΔVsyst).
In this regard, biological aortic grafts [29, 30] produced using special technologies from major vessels of cattle, with preserved structure of collagen and elastin fibers, provide an advantage regarding mechanical distensibility and elasticity. Currently, these are the only grafts capable of maintaining the wall distensibility of a complex hemodynamic structure such as the aorta [30]. MR elastometry can be used for aortic elasticity monitoring after replacement, with the desired frequency and duration of follow-up [8]. It can be used to assess the aortic wall and mechanical distensibility parameters in patients with ascending aortic replacement and in experimental settings.
CONCLUSION
MR elastometry provides therapeutically valuable information when used for the quantitative assessment of the biomechanical state of the ascending aorta. This should be considered when conducting studies in patients with aortic diseases, both atherosclerotic and due to other causes, and during prosthetic graft replacement of the ascending aorta irreversibly changed due to aneurysm.
MR elastometry with ECG gating for assessing the distensibility and calculating the Young’s modulus for the damaged aortic wall currently has no alternatives, because X-ray computed tomography-based elastometry, which is methodologically equivalent, is inherently associated with radiation exposure.
MR elastometry is expected to gain more clinical use, as calculating the systolic expansion volume of the aorta is clinically relevant in other aortic biomechanics and coronary circulation disorders of various origins.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. A.V. Friedman — development of a research plan, data processing, clinical evaluation of MRI results, writing text, editing text, preparing illustrations for the article; T.A. Bergen — development of the work plan, clinical evaluation of MRI results, text editing, final approval of the publication version of the article; D.A. Sirota — development of the work plan, clinical evaluation of MRI results and evaluation of cardiac surgery results, final approval of the publication version of the article; B.N. Kozlov — performing cardiac surgery and evaluating its results, editing the text; I.Y. Zhuravleva — participation in the development of the work concept, text editing, final approval of the publication version of the article; A.R. Tarkova — clinical evaluation of MRI results, writing the text, preparing illustrations for the article; W.Yu. Ussov — development of the work concept, data processing, clinical evaluation of MRI results, writing the text, preparation of illustrations for the article, final approval of the publication version of the article; A.M. Chernyavsky — development of the work concept, clinical evaluation of MRI results, text editing, final approval of the publication version of the article.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics Journal.
Fig. 2. Magnetic resonance imaging with ECG gating in patient B-k: (a) magnetic resonance angiography of the thoracic aorta. The lumen at the supravalvular and aortic arch levels and the distances between them, which are used to calculate the ascending aorta volume during systole and diastole and the systolic expansion volume, are shown. The turquoise horizontal line with arrows at the ends represents the tomographic slice level; (b) transverse tomographic slice of the ascending aorta in the wall area, with thickness measurements for the subsequent calculations of Young’s modulus parameters. The measurements are marked by thin green lines, with respective values.
Fig.1. Transverse slices of T1-weighted images of the chest organs, including the thoracic aorta, at the pulmonary artery bifurcation level in patient B-k: (a) before prosthetic replacement of the thoracic aorta expanded due to aneurysm; critical ascending aortic aneurysm with a >6 cm lumen expansion; (b) after prosthetic replacement of the thoracic aorta expanded due to aneurysm; normal lumen of the ascending aorta. The descending aorta was normal before and after surgery. The pulmonary artery expanded to 27 mm after surgery, compared to 23 mm at admission. Postoperative tomography revealed an artifact in the chest area due to a wire fixator.
Fig. 3. Cross-sectional dimensions and areas of the ascending aorta during systole and diastole: top row: baseline (at admission; before replacement of the aorta that expanded due to aneurysm); bottom row: after replacement with a synthetic graft; (a, c) diastole; (b, d) systole. Of note is a considerable lumen narrowing after surgery, with a relatively small distensibility of the ascending aorta.
About the authors
Alexander V. Friedman
E. Meshalkin National Medical Research Center
Email: fridman_av@meshalkin.ru
ORCID iD: 0000-0002-2300-2418
SPIN-code: 9508-8975
MD
Russian Federation, NovosibirskTatiana A. Bergen
E. Meshalkin National Medical Research Center
Email: tbergen@yandex.ru
ORCID iD: 0000-0003-1530-1327
SPIN-code: 5467-7347
MD, Dr. Sci. (Medicine)
Russian Federation, NovosibirskDmitry A. Sirota
E. Meshalkin National Medical Research Center
Email: d_sirota@meshalkin.ru
ORCID iD: 0000-0002-9940-3541
SPIN-code: 4706-7549
MD, Cand. Sci. (Medicine)
Russian Federation, NovosibirskBoris N. Kozlov
Cardiology Research Institute of the Tomsk National Research Medical Center
Email: kbn@cardio-tomsk.ru
ORCID iD: 0000-0002-0217-7737
SPIN-code: 9265-9432
MD, Dr. Sci. (Medicine)
Russian Federation, TomskIrina Yu. Zhuravleva
E. Meshalkin National Medical Research Center
Email: zhuravleva_i@meshalkin.ru
ORCID iD: 0000-0002-1935-4170
SPIN-code: 7322-1480
MD, Dr. Sci. (Medicine), Professor
Russian Federation, NovosibirskAlexandra R. Tarkova
E. Meshalkin National Medical Research Center
Email: a_tarkova@meshalkin.ru
ORCID iD: 0000-0002-4291-6047
SPIN-code: 8547-4380
MD, Cand. Sci. (Medicine)
Russian Federation, NovosibirskWladimir Yu. Ussov
E. Meshalkin National Medical Research Center
Author for correspondence.
Email: ussov1962@yandex.ru
ORCID iD: 0000-0001-7978-5514
SPIN-code: 1299-2074
MD, Dr. Sci. (Medicine), Professor
Russian Federation, NovosibirskAlexander M. Chernyavskiy
E. Meshalkin National Medical Research Center
Email: a_cherniavsky@meshalkin.ru
ORCID iD: 0000-0001-9818-8678
SPIN-code: 5286-6950
MD, Dr. Sci. (Medicine), Professor, Corresponding Member of the Russian Academy of Sciences
Russian Federation, NovosibirskReferences
- Bokeriya LA, Malashenkov AI, Rusanov NI, et al. Surgical treatment of ascending aortic aneurysm with concomitant coronary artery disease. Annaly khirurgii. 2004(2):35–42. (In Russ).
- Konstantinov BA, Belov YuV, Kuznechevskii FV. Aneurysm of the ascending aorta and aortic arch. Moscow: Astrel’; 2006. (In Russ).
- Belov IuV, Isaev PM. Modern strategies of surgical treatment of aortic arch aneurysms. Pirogov Russian Journal of Surgery (Khirurgiya. Zurnal im. N.I Pirogova). 2014(10):122–126.
- Sirota DA, Zhulkov МО, Khvan DS. Predictors of Lethality, Remodeling, and Aorta-Related Events in Different Types of Proximal Aortic Dissection Surgery. Modern Technologies in Medicine. 2023;15(1):38–52. doi: 10.17691/stm2023.15.1.05
- Belov IuV, Isaev RM. Risk stratification in cardiovascular surgery. Pirogov Russian Journal of Surgery (Khirurgiya. Zurnal im. N.I. Pirogova). 2014(7):78–81.
- Karpman VL, Orel VR. Arterial system impedance and cardiac function. Human Physiology. 1985;(4):628–633. (In Russ).
- Zhuravleva IYu, Lyashenko MM, Shadanov AA, Sirota DA, Chernyavskiy AM. Quo vadimus? Fundamental problems of developing hybrid prostheses of thoracic aorta. Angiology and vascular surgery. 2021;27(4):103–112. doi: 10.33529/ANGIO2021412
- Ussov WYu, Ignatenko GA, Bergen TA, et al. Computational evaluation of mechano-elastic properties and of paramagnetic contrast enhancement of thoracic aortic wall in acute myocardial infarction and in non-coronarogenic myocardial damage, from the data of dynamic ECG-gated MRI (MR-elastometry). Translational Medicine. 2021;8(6):43–58. doi: 10.18705/2311-4495-2021-6-43-58
- Ussov WYu, Igantenko GA, Maksimova AS, et al. The relationship of structural changes in the wall of the ascending aorta and myocardium according to chest contrast-enhanced MRI in myocardial infarction patients. Regional blood circulation and microcirculation. 2023;22(1):41–51. doi: 10.24884/1682-6655-2023-22-1-41-51
- Purinya BA, Kas’yanov VA. Biomechanics of human large blood vessels. Riga: Zinatne; 1980. (In Russ).
- Karo K, Pedli T, Shroter R, Sid U. Circulatory mechanics. Moscow: Mir; 1981. (In Russ).
- Skripnik AYu, Fokin VA, Mironchuk RR, et al. Assessment of the elastic properties of the ascending aorta using electrocardiographic synchronized computed tomography angiography with advanced data processing. Russian Journal of Cardiology. 2019;24(12):48–54. doi: 10.15829/1560-4071-2019-12-48-54
- Zel’dovich YaB. Advanced Math for Beginners. Moscow: Nauka; 1963. (In Russ).
- Dudko VA, Karpov RS. Atherosclerosis of heart and brain vessels. Tomsk: STT; 2002. (In Russ).
- Kolipaka A, Woodrum D, Araoz PA, Ehman RL. MR elastography of the in vivo abdominal aorta: a feasibility study for comparing aortic stiffness between hypertensives and normotensives. J Magn Reson Imaging. 2012;35(3):582–586. doi: 10.1002/jmri.22866
- Damughatla AR, Raterman B, Sharkey-Toppen T, et al. Quantification of aortic stiffness using MR elastography and its comparison to MRI-based pulse wave velocity. J Magn Reson Imaging. 2015;41(1):44–51. doi: 10.1002/jmri.24506
- Kolipaka A, Araoz PA, McGee KP, Manduca A, Ehman RL. Magnetic resonance elastography as a method for the assessment of effective myocardial stiffness throughout the cardiac cycle. Magn Reson Med. 2010;64(3):862–870. doi: 10.1002/mrm.22467
- Dresner MA, Rose GH, Rossman PJ, et al. Magnetic resonance elastography of skeletal muscle. J Magn Reson Imaging. 2001;13(2):269–276. doi: 10.1002/1522-2586(200102)13:2<269::aid-jmri1039>3.0.co;2-1
- Hrabak-Paar M, Kircher A, Al Sayari S, et al. Variability of MRI Aortic Stiffness Measurements in a Multicenter Clinical Trial Setting: Intraobserver, Interobserver, and Intracenter Variability of Pulse Wave Velocity and Aortic Strain Measurement. Radiol Cardiothorac Imaging. 2020;2(2):e190090. doi: 10.1148/ryct.2020190090
- Woodrum DA, Romano AJ, Lerman A, et al. Vascular wall elasticity measurement by magnetic resonance imaging. Magn Reson Med. 2006;56(3):593–600. doi: 10.1002/mrm.20991
- Kobelev E, Shadanov AA, Sirota DA, et al. Volumetric analysis on computed tomography Angiography in the management of thoracic aortic dissection in case of seven years follow-up period. Medical Visualization. 2022;26(3):46–56. doi: 10.24835/1607-0763-1060
- Kobelev E, Bergen TA, Tarkova AR, et al. A New Look at Structural Changes in the Aortic Root in Aortic Valve Stenosis. Modern Technologies in Medicine. 2022;14(2):51–58. doi: 10.17691/stm2022.14.2.05
- Nepomnyashchikh LM. Morphogenesis of the most important common pathologic processes in the heart. Novosibirsk: Nauka; 1991. (In Russ).
- Ussov WYu, Belichenko OI, Maksimova AS, et al. Magnetic resonance imaging of the aortic wall with paramagnetic contrast enhancement in assessing the severity of its atherosclerotic lesion and predicting occlusive thrombotic arterial complications. Terapevt. 2017;128(9):55–62. (In Russ).
- Badji A, Sabra D, Bherer L, et al. Arterial stiffness and brain integrity: A review of MRI findings. Ageing Res Rev. 2019;53. doi: 10.1016/j.arr.2019.05.001
- Lechner I, Reindl M, Tiller C, et al. Determinants and prognostic relevance of aortic stiffness in patients with recent ST-elevation myocardial infarction. Int J Cardiovasc Imaging. 2022;38(1):237–247. doi: 10.1007/s10554-021-02383-0
- Pribylov SA, Yakovleva MV, Pribylov VS, et al. Arterial stiffness in patients with acute coronary syndrome without persistent ST segment elevation combined with chronic kidney disease and arterial hypertension and its correction with antihypertensive therapy. Humans and their health. 2022;25(1):19–27. doi: 10.21626/vestnik/2022-1/03
- Soynov IA, Zhuravleva IY, Kulyabin YY, et al. Tissue Engineering in Cardiovascular Surgery: Evolution and Contemporary Condition of the Problem. Journal of Experimental and Clinical Surgery. 2019;12(1):71–80. doi: 10.18499/2070-478X-2019-12-1-71-80
- Zhuravleva IYu, Timchenko TP, Vladimirov SV, et al. Ab ovo: Factors Affecting the Radial Stiffness of Thoracic Aorta Stent-Grafts. Modern Technologies in Medicine. 2021;13(1):17–26. doi: 10.17691/stm2021.13.1.02
- Vasilyeva MB, Kuznetsova EV, Rusakova YaL, et al. Mechanical properties of native and decellularized aortic wall after long-term storage in biocide solutions. Russian Journal of Transplantology and Artificial Organs. 2021;23(4):86–94. doi: 10.15825/1995-1191-2021-4-86-94
Supplementary files