Conventional structural magnetic resonance imaging in differentiating chronic disorders of consciousness
- Authors: Sergeeva A.N.1, Morozova S.N.1, Sergeev D.V.1, Kremneva E.I.1, Zimin A.A.1, Legostaeva L.A.1, Iazeva E.G.2, Krotenkova M.V.1, Ryabinkina Y.V.1, Suponeva N.A.1, Piradov M.A.1
-
Affiliations:
- Research Center of Neurology
- LLC “Three sisters” Rehabilitation center
- Issue: Vol 5, No 2 (2024)
- Pages: 190-202
- Section: Original Study Articles
- Submitted: 15.09.2023
- Accepted: 04.12.2023
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/569418
- DOI: https://doi.org/10.17816/DD569418
- ID: 569418
Cite item
Abstract
BACKGROUND: Differential diagnosis of chronic disorders of consciousness remains one of the most difficult problems even for experienced clinicians.
AIM: To evaluate the inter-expert consistency and capacity of the researcher-developed structural scale based on magnetic resonance imaging to differentiate chronic disorders of consciousness, named, DOC-MRIDS, on a larger sample of patients.
MATERIALS AND METHODS: Sixty patients with a clinically stable status diagnosed with consciousness disorders (vegetative state, n=32; minimally conscious state, n=28) were enrolled. The revised coma recovery scale (CRS-R) was included in the clinical assessment. All patients underwent structural magnetic resonance imaging with 3.0-T Siemens scanners including T2 and T1 sequences. Structural changes were assessed using the DOC-MRIDS scale and included the following features: diffuse cortical atrophy, ventricular enlargement, gyri dilatation, leukoaraiosis, brainstem and/or thalamic degeneration, corpus callosum degeneration, and focal corpus callosum lesions. A total score was calculated. Magnetic resonance imaging data were analyzed by three neuroradiologists, and inter-observer agreement (Krippendorf’s alpha) was assessed.
RESULTS: A high inter-examiner agreement of the DOC-MRIDS scale score was found, with α=0.806 (95% confidence interval 0.757–0.849). The vegetative state group had a higher DOC-MRIDS score than the minimally conscious state group (p <0.005). A negative correlation was obtained between CRS-R and DOC-MRIDS scale scores (ρ=–0.457, p <0.0001), individual clinical scale domains, and magnetic resonance imaging features.
CONCLUSION: When assessing structural changes in patients with chronic consciousness disorders, the use of the DOC-MRIDS scale helps differentiate the type of such disorders with sufficient specificity, sensitivity, and inter-rater agreement. This scale can be used in clinical practice as an additional differential diagnostic tool.
Full Text
BACKGROUND
Chronic disorders of consciousness (CDoC), defined as the chronic failure to regain conscious behavior after a coma despite restored wakefulness, are typically caused by extensive damage to several brain structures [1]. In CDoC, the vegetative functions of the hypothalamus and brain stem (specifically circulatory control and spontaneous breathing) are either fully or partially preserved, allowing patients to survive for extended periods [2]. The two main types of CDoC are vegetative state (VS, or unresponsive wakefulness syndrome) and minimally conscious state (MCS) [2, 3]. VS is a clinical condition characterized by a complete absence of awareness of oneself and the environment. In contrast, patients in an MCS demonstrate unstable but distinct and reproducible behavioral signs of self-awareness or responses to their surroundings [3, 4].
In the differential diagnosis of VS and MCS, identifying signs of consciousness is crucial, which largely determines the subsequent rehabilitation strategy [3]. Diagnostic criteria for distinguishing between VS and MCS include clinical signs of conscious responses to external stimuli and the possibility of purposeful communication. A detailed, standardized clinical examination remains the gold standard for detecting signs of consciousness [5]. The Coma Recovery Scale-Revised (CRS-R) is widely used to assess CDoC patients. This scale provides optimal results for the differential diagnosis of VS and MCS [6]. However, its use is frequently challenging due to the complex interpretation of observed responses, variations in the patient’s activity level, severe motor deficiency, aphasia, mechanical obstacles (such as a tracheostomy tube), pain syndrome, and various other factors. As a result, the diagnosis error rate can reach 40% [7].
Numerous studies have been performed to improve the differential diagnosis of CDoC and predict the outcomes using neuroimaging techniques, such as brain positron emission tomography with 18F-fludeoxyglucose. This method has been shown to complement clinical evaluations and help predict the long-term recovery of CDoC patients [8]. Monti et al. demonstrate brain activation in a small proportion of patients using functional magnetic resonance imaging (fMRI) when performing specific mental imagery tasks, which also implies the presence of consciousness [9]. Another study found a correlation between the restoration of frontal-thalamic interactions according to resting-state fMRI and the recovery of cognitive function [10]. Moreover, another study [11] that evaluated the functional connectivity of resting-state networks (default mode network, frontoparietal network, salience network, auditory network, sensorimotor network, and visual network) found differences between VS and MCS patients in more than 80% of cases.
However, functional imaging techniques are not widely used because they require complex data processing, and the findings can be conflicting. Structural imaging is a far more accessible tool for determining the severity of brain injury in CDoC. In a large patient sample (n = 143), this method has demonstrated that CDoC is characterized by severe atrophy of basal nuclei and the thalamus [12]. Moreover, several attempts were made to identify brain injury patterns that could help distinguish between VS and MCS patients. For example, studies have shown that patients in a VS had more severe atrophy of the left putamen and globus pallidus, decreased volume of the thalamus, and more pronounced changes in the ventromedial prefrontal cortex, posterior cingulate cortex, and precuneus compared to MCS patients. Furthermore, MCS subtypes were examined based on the intricacy of the observed behavioral response: MCS–and MCS+. In MCS+ patients, the left cerebral cortex was more intact, including the middle temporal gyrus, superior temporal gyrus, and inferior frontal gyrus (the Broca area) [13]. The prognostic value of structural MRI findings in posttraumatic VS patients has been confirmed: damage to the corpus callosum and dorsolateral brain stem were predictors of failure to recover. Additionally, a study using a morphometric approach demonstrated differences between VS and MCS patients in terms of gray matter volume in the paracentral, parahippocampal, inferior parietal, and medial orbitofrontal cortex, as well as thalami and caudate nuclei [14].
However, complex analysis of imaging findings may not be available in routine practice. Considering the wide use of structural MRI, its necessity in the majority of CDoC patients, and the confirmed association between MRI changes and the clinical presentation in CDoC patients, the Research Center of Neurology developed the Disorders of Consciousness MRI-Based Distinguishing Scale (DOC-MRIDS) [15]. This scale evaluates the most common and clinically significant MRI changes reported in CDoC patients, providing further evidence for the differential diagnosis of VS and MCS. The scale was tested in 30 patients and demonstrated high sensitivity and specificity (82% and 92%, respectively); however, its wider clinical use requires further reliability assessment.
STUDY AIM
This study aimed to evaluate the potential of DOC-MRIDS for the differential diagnosis of CDoC in a larger sample of patients and to determine the inter-rater consistency of assessment findings.
MATERIALS AND METHODS
Study Design
This was an experimental, single-center, cross-sectional study.
Eligibility Criteria
The inclusion criteria include the following:
- confirmed CDoC;
- age above 18 years;
- time from the onset of disorder of consciousness of at least 28 days;
- stable somatic condition;
- informed consent of the patient’s legal representative.
Patients with contraindications to MRI were excluded.
Investigational Site
The study was performed in the intensive care unit of the Research Center of Neurology (Moscow).
Study Duration
Study subjects were enrolled between 2015 and 2021.
Therapeutic Intervention
All patients underwent structural MRI using MAGNETOM Verio 3T и MAGNETOM Prisma 3T scanners (Siemens Healthineers, Germany). The imaging protocol included T2- weighted images using a spin echo sequence (repetition time: 4,000 msec; echo time: 118 msec; slice thickness: 5.0 mm; slice spacing: 1.5 mm; duration: 2 min 02 sec) and Т1-weighted images using a 3D gradient echo sequence (repetition time: 1,900 ms; echo time: 2.5 msec; slice thickness: 1.0 mm; slice spacing: 1.0 mm; slice number: 176; duration: 4 min 18 sec) to assess the brain matter.
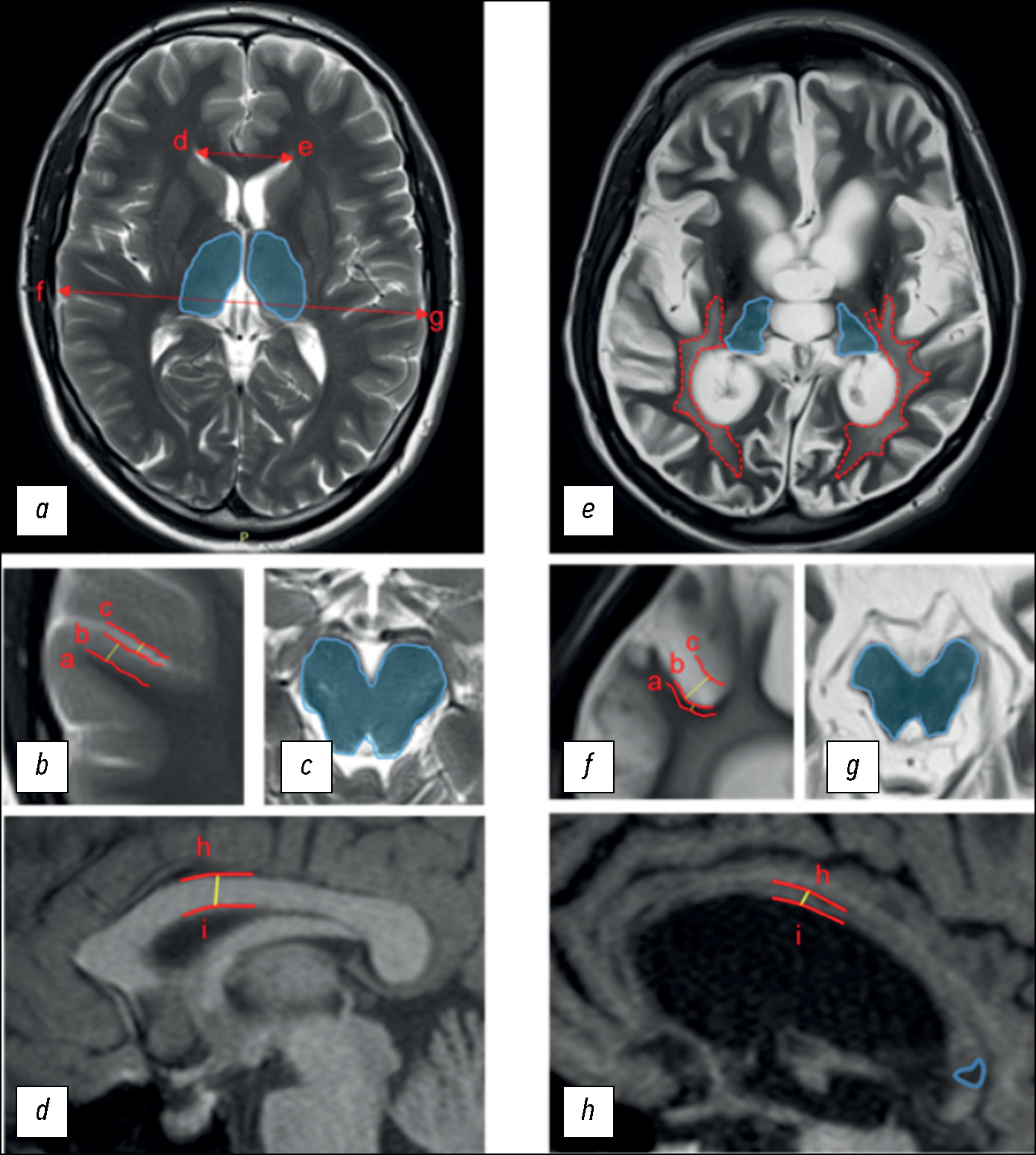
To assess structural changes according to DOC-MRIDS, the most commonly reported indicators were selected, including diffuse cortical atrophy, ventricular enlargement, sulcus widening, leukoaraiosis, degeneration of the brain stem and/or thalamus, corpus callosum degeneration, and focal lesion of the corpus callosum (Fig. 1).
Fig. 1. Disorders of Consciousness MRI-Based Distinguishing Scale (DOC-MRIDS) score: (a–d) in a healthy volunteer; (e–h) in a CDoC patient. Specified distances: (a–b) cortex thickness; (b–c) sulcus width; (h–i) thickness of the central part of the corpus callosum; distances (d–e; f–g) were used to calculate the Evans index. Blue areas: (a) unchanged thalami; (c) brain stem; (e) thalamic degeneration; (g) brain stem degeneration. Lines: (e) red dotted lines represent the extent of leukoaraiosis; (h) the solid blue line corresponds to hypointense lesions in the corpus callosum.
In diffuse cortical atrophy, brain stem and/or thalamus degeneration, and corpus callosum damage, the changes were classified as the presence (1) or absence (0) of pathology. Confirmed extensive cortical thinning and symmetrical bilateral decrease in thalamic and/or brain stem size were considered. Unilateral damage due to stroke or injury was considered to be the absence of the sign. The remaining parameters were assessed as follows: 0 = no changes; 1 = moderate changes; 2 = severe changes:
- moderate and severe ventricular enlargement corresponded to the Evans index of 0.31 to 0.74 (1) and >0.74 (2), respectively;
- subarachnoid space enlargement of 0.2–0.4 cm was classified as moderate sulcus widening (1) and of >0.4 cm as severe sulcus widening (2);
- periventricular caps and diffuse periventricular changes in the signal intensity in the white matter were classified as moderate leukoaraiosis (1); large, confluent T2 hyperintense areas extending to deep white matter and the subcortex were classified as severe leukoaraiosis (2);
- corpus callosum degeneration was assessed by the thickness of its central part: 0.4–0.2 cm for moderate degeneration (1) and <0.2 cm for severe degeneration (2).
Following a visual assessment of the aforementioned changes, the total score was calculated on a scale of 0 (unchanged brain) to 11 (extensive brain injury) (Table 1).
Table 1. Magnetic resonance imaging parameters for assessment according to Disorders of Consciousness Magnetic Resonance Imaging-Based Distinguishing Scale
Parameters | DOC-MRIDS | |||
Absence | Presence | Moderate changes | Severe changes | |
Diffuse cortical atrophy | 0 | 1 | – | – |
Brain stem/thalamus degeneration | 0 | 1 | – | – |
Focal lesion of the corpus callosum | 0 | 1 | – | – |
Ventricular enlargement | 0 | – | 1 | 2 |
Sulcus widening | 0 | – | 1 | 2 |
Leukoaraiosis | 0 | – | 1 | 2 |
Corpus callosum degeneration | 0 | – | 1 | 2 |
DOC-MRIDS, Disorders of Consciousness MRI-Based Distinguishing Scale
All these MRI parameters were independently assessed based on T2- and T1-weighted images by three neuroradiologists (work experience over 15 years) who were blinded to the clinical diagnosis.
Three neurologists (with at least three years of experience in the treatment of CDoC patients) conducted the clinical assessment. They made the diagnosis of VS or MCS using a validated version of the CRS-R in Russian [4]. The assessment was performed at least five times, and the best result was used.
Subgroup Analysis
The study included 60 patients (28 women and 32 men) aged 18 to 67 years (median age: 32 [24; 49] years) with a clinical diagnosis of CDoC (VS: n = 31; MCS: n = 29). All patients were in a stable somatic condition.
Ethics Review
All legal representatives of patients provided informed consent. All procedures involving humans in this study adhered to the ethical standards of the Institutional and/or National Research Committee and the 1964 Declaration of Helsinki, as amended, or comparable ethical standards. The informed consent protocol was approved by the Ethics Committee of the Research Center of Neurology on November 19, 2014 (project ID: 11/14).
Statistical Analysis
When calculating the sample size according to a well-established algorithm, it was shown that a sample size of 60 patients is sufficiently representative. The data distribution in the sample (n = 60) was non-normal; thus, nonparametric statistical methods were used for the analysis, such as the Kruskal–Wallis test and the Mann–Whitney U test, to compare patients across three or more parameters, the Benjamini–Hochberg multiplicity adjustment was applied. The nonparametric Spearman’s correlation coefficient was used to assess the association between parameters. The chi-squared test with continuity correction was used to analyze categorical variables. Receiver operating characteristic (ROC) analysis was used to assess the informative value of the scale by calculating the area under the curve (AUC), as well as sensitivity and specificity parameters.
The inter-rater consistency was assessed using Krippendorff’s alpha. The significance level for all hypothesis tests corresponded to P < 0.05.
The descriptive statistics results are presented as median (Me) and interquartile range (Q25–Q75). The statistical analysis was performed using SPSS Statistics 22 (IBM, Chicago, IL, USA) and Rstudio (Posit PBC, Boston, MA, USA).
RESULTS
Study Subjects
The duration of CDoC was 2 to 72 months after emerging from coma. CDoC was due to a traumatic brain injury (TBI; n = 19) and non-traumatic causes (anoxic brain injury, acute ischemic or hemorrhagic cerebrovascular accident, demyelinating disease, intoxication, etc.; n = 41).
Table 2 summarizes demographic and clinical data for VS and MCS patients. The median total CRS-R score in VS and MCS patients was 6 and 12 points, respectively.
Table 2. Demographic and clinical data
Vegetative state | Minimally conscious state | All patients | |
Number | 31 | 29 | 60 |
Age, years | 34 [24; 51] | 32 [25; 45] | 32 [24; 49] |
Sex, male/female | 15/16 | 17/12 | 28/32 |
Traumatic brain injury/non-traumatic causes | 4/27* | 15/14* | 19/41 |
Duration of the disease, months | 14 (2–72) | 12 (2–56) | 13 (2–72) |
DOC-MRIDS | 4** | 6** | 5,5 |
CRS-R | 6* | 12,0* | 9 |
CRS-R, Coma Recovery Scale-Revised; DOC-MRIDS, Disorders of Consciousness MRI-Based Distinguishing Scale; *P < 0.05; **P < 0.005 (Mann–Whitney U test).
Primary Findings
There were no significant differences between VS and MCS patients in terms of age (Mann–Whitney U test, P = 0.982), disease duration (P = 0.807), and sex (chi-squared test with continuity correction, P = 0.453). Non-TBI in VS patients was significantly more common than TBI (chi-squared test, P = 0.003), while MCS patients had a similar incidence of non-traumatic and TBI (chi-squared test, P = 0.268). In general, the clinical presentation in patients with non-TBI was more severe than in patients with TBI. The median total CRS-R score was 6.0 (5.0–10.0) and 11.0 (8.0–16.0), respectively (Mann–Whitney U test with the Benjamini–Hochberg multiplicity adjustment, P = 0.001).
Three experts demonstrated strong inter-rater consistency when assessing images according to DOC-MRIDS, as measured by Krippendorff’s alpha: α = 0.806 (95% confidence interval: 0.757–0.849). The Kruskal–Wallis test was used to select findings from one of the three experts. There were no significant differences between the experts (P = 0.363); hence, the findings of the second experts were used for further analysis.
When assessing the association between the duration of CDoC and the DOC-MRIDS score, there was a weak positive correlation (Spearman’s correlation coefficient 0.338; P = 0.008). Spearman’s correlation analysis showed a significant negative correlation between the CRS-R and DOC-MRIDS scores (ρ = –0.457, P < 0.0001) (Fig. 2). Moreover, significant negative correlations were observed between the total CRS-R score and the following parameters: diffuse cortical atrophy (ρ = –0.457); lesions in the corpus callosum (ρ = 0.349); ventricular enlargement (ρ = –0.342); sulcus widening (ρ = 0.442); and leukoaraiosis (ρ = 0.502); P < 0.001 in all cases. There was also a significant correlation between individual CRS-R domains and nearly all structural scale parameters (Table 3).
Fig. 2. Negative correlation between the Coma Recovery Scale-Revised (CRS-R) and Disorders of Consciousness MRI-Based Distinguishing Scale (DOC-MRIDS) scores (ρ = –0.457, P < 0.0001). Red dots: vegetative state group; blue dots: minimally conscious state group.
Table 3. Correlation coefficients between individual the Coma Recovery Scale-Revised domains and Disorders of Consciousness Magnetic Resonance Imaging-Based Distinguishing Scale parameters
Statistical parameter | Auditory function | Visual function | Motor function | Oromotor function | Communication | Wakefulness |
Diffuse cortical atrophy | ||||||
Correlation coefficient (ρ) | –0.472** | –0.382** | –0.492** | –0.152 | –0.315* | –0.159 |
P-value | 0.000 | 0.005 | 0.000 | 0.278 | 0.022 | 0.255 |
Brain stem and/or thalamus degeneration | ||||||
Correlation coefficient (ρ) | –0.288* | –0.212 | –0.209 | –0.091 | –0.140 | –0.137 |
P-value | 0.035 | 0.124 | 0.130 | 0.513 | 0.314 | 0.323 |
Lesions in the corpus callosum | ||||||
Correlation coefficient (ρ) | 0.289* | 0.360** | 0.355** | 0.229 | 0.164 | 0.142 |
P-value | 0.032 | 0.007 | 0.008 | 0.093 | 0.232 | 0.301 |
Ventricular enlargement | ||||||
Correlation coefficient (ρ) | –0.276* | –0.318* | –0.379** | –0.149 | –0.309* | –0.256 |
P-value | 0.041 | 0.018 | 0.004 | 0.276 | 0.022 | 0.059 |
Sulcus widening | ||||||
Correlation coefficient (ρ) | –0.502** | –0.425** | –0.516** | –0.169 | –0.341* | –0.201 |
P-value | 0.000 | 0.001 | 0.000 | 0.218 | 0.011 | 0.141 |
Leukoaraiosis | ||||||
Correlation coefficient (ρ) | –0.405** | –0.451** | –0.480** | –0.338* | –0.396** | –0.250 |
P-value | 0.002 | 0.001 | 0.000 | 0.012 | 0.003 | 0.066 |
Corpus callosum atrophy | ||||||
Correlation coefficient (ρ) | –0.165 | –0.135 | –0.049 | –0.174 | –0.001 | –0.141 |
P-value | 0.229 | 0.324 | 0.722 | 0.204 | 0.996 | 0.305 |
*P < 0.05; **P < 0.005 (Spearman’s correlation analysis).
Additionally, statistical between-group differences were observed according to DOC-MRIDS. For example, VS patients had a lower DOC-MRIDS score compared to MCS patients (Mann–Whitney U test, P < 0.005). According to ROC analysis in this sample, the threshold DOC-MRIDS score for distinguishing between VS and MCS patients was 5.5, with an optimal sensitivity of 68% and a specificity of 64% (AUC = 0.71; P = 0.005; Fig. 3).
Fig. 3. ROC curve for distinguishing between vegetative state and minimally conscious state patients (AUC = 0.71; P = 0.005). ROC, receiver operating characteristic
There were significant differences between causative factors. Signs, such as cortical atrophy (chi-squared test, P < 0.0001), sulcus widening (P = 0.001), and leukoaraiosis, were significantly more common in patients with non-TBI (P = 0.004), whereas patients with traumatic CDoC had a higher incidence of lesions in the corpus callosum (P = 0.002).
DISCUSSION
Summary of Primary Findings
The MRI scale for quantitative assessment of structural brain changes characteristic of CDoC was proposed as an additional tool for the differential diagnosis of clinical variants of CDoC. A pilot study demonstrated that the scale can be used in routine practice with sufficiently high sensitivity and specificity [15]. This study was the next step in the scale’s development and attempted to assess its reliability when used by different radiologists and in a larger sample of patients.
Discussion of Primary Findings
The findings obtained in a twofold larger patient population balanced by the CDoC type show that DOC-MRIDS has a lower sensitivity and specificity than the pilot study: 82% and 92% vs. 68% and 64%, respectively. This could be related to a considerable variation in the duration of CDoC (2–72 months). However, these values are high enough to use the scale in clinical practice. A high inter-rater consistency indicates that reliable results can be obtained when different radiologists perform the assessment.
Notably, the assessment included data from clinically stable patients with no somatic conditions that could compromise the results of neurological consciousness assessment (such as infectious diseases, elevated intracranial pressure, etc.). Moreover, clinical data on the state of consciousness were obtained by repeated evaluation using the most reliable scale, CRS-R; the assessment was performed by neurologists with sufficient experience in the treatment of CDoC patients [16, 17]. This approach ensures the most accurate diagnosis of CDoC type. Furthermore, inconsistencies between X-ray findings according to DOC-MRIDS and the clinical presentation in CDoC patients may indicate the need for repeated neurological assessment in some cases.
Notably, most VS patients had non-traumatic disorders of consciousness. This reflects the challenges in enrolling CDoC patients: the prognosis after TBI is somewhat better, and this group of patients develops MCS more frequently. As a result, it would take longer to enroll VS patients with non-TBI and form a population balanced by the causative factor. This characteristic of the study group could affect the interpretation of assessment according to DOC-MRIDS because patients with non-TBI had more severe changes in such parameters as cortical atrophy, sulcus widening, and leukoaraiosis. Thus, further assessment of the effect of the causative factor on the DOC-MRIDS score in VS patients requires a study in a larger sample of patients, balanced by the number of traumatic and non-traumatic brain injuries.
A weak positive correlation between the duration of CDoC and the DOC-MRIDS score may reflect morphological changes over time. There have been no detailed long-term studies on this subject.
DOC-MRIDS has an undeniable advantage in terms of availability because the assessment requires structural MRI findings (T2-weighted spin echo sequence and Т1-weighted gradient echo sequence with the specified imaging parameters), which can be obtained using any high-field scanner with a magnetic field intensity above 1.0 T.
Patient motion artifacts are a common problem in MRI examinations, making the assessment, according to DOC-MRIDS, challenging, especially for MCS patients. Mitigating the artifacts may require sedation, which is associated with potential risks for patients and makes the study more challenging to conduct. However, when using structural MRI, special image reconstruction algorithms can minimize the effect of artifacts. Furthermore, structural MRI findings, including the DOC-MRIDS score, are substantially less susceptible to the impact of motion artifacts compared to functional MRI, emphasizing the availability of this method in routine practice.
Our study is one of the first to incorporate morphological signs of brain injury in CDoC patients into a single scale. Our findings are consistent with some previous studies in patients with early posttraumatic brain injuries causing disorders of consciousness. For example, it was shown that traumatic injuries of the corpus callosum and dorsolateral brain stem are predictors of failure to recover and may result in unfavorable outcomes [14]. In our study, severe corpus callosum atrophy and brain stem and thalamus degeneration were most commonly observed in VS patients.
Individual domains and the total CRS-R score significantly correlated with the total DOC-MRIDS score and changes in its individual parameters, indicating the significance of the morphological integrity of anatomical structures included in the consciousness assessment scale [18]. Some brain areas may contribute to consciousness formation and maintenance, but their structural integrity alone is insufficient for clear consciousness formation. Moreover, the maintenance of consciousness requires the integrity of thalamocortical interactions and interactions within and between various brain networks [19]. This may explain the observed strongest correlations between individual CRS-R domains and diffuse changes in the white matter and focal lesions of the corpus callosum, which form the structural basis for intracerebral interactions. However, automatic group analysis of data on brain connectivity in CDoC patients collected using structural and functional MRI is difficult due to high variability and severe changes in the brain matter. Despite advances in CDoC studies, recent findings [19–21] highlight the need for more extensive, simultaneous examination of structural and functional characteristics of the brain in CDoC patients, as well as the development of computational data processing methods, including using artificial intelligence [20].
CONCLUSION
A comprehensive analysis of structural changes in CDoC patients, along with their attempted quantitative assessment using the DOC-MRIDS scale, helps to determine the likely clinical variant of CDoC with sufficient specificity, sensitivity, and inter-rater consistency. This method can be used in clinical practice in addition to the findings of a structured neurological examination.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
The greatest contribution is distributed as follows: A.N. Sergeeva — review of publications on the topic of the article, processing of MRI data, analysis of the obtained results, writing the manuscript text; S.N. Morozova — review of publications on the topic of the article, processing of MRI data, writing the manuscript text; D.V. Sergeev — methodology, scientific advice, review of publications on the topic of the article, reviewing and editing of the manuscript text; E.I. Kremneva — methodology, MRI data collection and processing; A.A. Zimin — statistical analysis of data, preparation of figures and tables; L.A. Legostaeva, E.G. Yazeva — methodology, collection and processing of clinical data; N.A. Suponeva, M.V. Krotenkova, M.A. Piradov — scientific consultation, project conception and administration, manuscript review.
About the authors
Anastasia N. Sergeeva
Research Center of Neurology
Author for correspondence.
Email: sergeeva@neurology.ru
ORCID iD: 0000-0002-2481-4565
SPIN-code: 6761-8250
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowSofya N. Morozova
Research Center of Neurology
Email: kulikovasn@gmail.com
ORCID iD: 0000-0002-9093-344X
SPIN-code: 2434-7827
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowDmitrii V. Sergeev
Research Center of Neurology
Email: dmsergeev@yandex.ru
ORCID iD: 0000-0002-9130-1292
SPIN-code: 8282-3920
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowElena I. Kremneva
Research Center of Neurology
Email: moomin10j@mail.ru
ORCID iD: 0000-0001-9396-6063
SPIN-code: 8799-8092
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowAlexey A. Zimin
Research Center of Neurology
Email: leha-zimin@inbox.ru
ORCID iD: 0000-0002-9226-2870
SPIN-code: 9525-1805
Russian Federation, Moscow
Lyudmila A. Legostaeva
Research Center of Neurology
Email: milalegostaeva@gmail.com
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowElizaveta G. Iazeva
LLC “Three sisters” Rehabilitation center
Email: lizaveta.mochalova@gmail.com
ORCID iD: 0000-0003-0382-7719
SPIN-code: 4895-3900
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowMarina V. Krotenkova
Research Center of Neurology
Email: krotenkova_mrt@mail.ru
ORCID iD: 0000-0003-3820-4554
SPIN-code: 9663-8828
MD, Dr. Sci. (Medicine)
Russian Federation, МоскваYulia V. Ryabinkina
Research Center of Neurology
Email: ryabinkina11@mail.ru
ORCID iD: 0000-0001-8576-9983
SPIN-code: 5044-2701
MD, Dr. Sci. (Medicine)
Russian Federation, MoscowNatalya A. Suponeva
Research Center of Neurology
Email: nasu2709@mail.ru
ORCID iD: 0000-0003-3956-6362
SPIN-code: 3223-6006
MD, Dr. Sci. (Medicine), corresponding member of the Russian Academy of Sciences, Professor
Russian Federation, MoscowMichael A. Piradov
Research Center of Neurology
Email: mpi711@gmail.com
ORCID iD: 0000-0002-6338-0392
SPIN-code: 2860-1689
MD, Dr. Sci. (Medicine), academician member of the Russian Academy of Sciences, Professor
Russian Federation, MoscowReferences
- Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17(5):307–321. doi: 10.1038/nrn.2016.22
- Monti MM, Laureys S, Owen AM. The vegetative state. BMJ. 2010;341:376–385. doi: 10.1136/bmj.c3765
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: Definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/wnl.58.3.349
- Belkin AA, Aleksandrova EV, Akhutina TV, et al. Chronic Disorders of Consciousness: guidelines of the All-Russian public organization “Federation of Anesthesiologists and Reanimatologists”. Annals of critical care. 2023;(3):7–42. doi: 10.21320/1818-474X-2023-3-7-42
- Giacino JT. The vegetative and minimally conscious states: Consensus-based criteria for establishing diagnosis and prognosis. Neurorehabilitation. 2004;19(4):293–298. doi: 10.3233/NRE-2004-19405
- Seel RT, Sherer M, et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil. 2010;91(12):1795–1813. doi: 10.1016/j.apmr.2010.07.218
- Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;(9):35–40. doi: 10.1186/1471-2377-9-35
- Stender J, Gosseries O, Bruno M, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet. 2014;384(9942):514–522. doi: 10.1016/S0140-6736(14)60042-8
- Monti M, Vanhaudenhuyse A, Coleman M, et al. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010;362(7):579–589. doi: 10.1056/NEJMoa0905370
- Crone J, Bio B, Vespa P, et. al. Restoration of thalamo-cortical connectivity after brain injury: Recovery of consciousness, complex behavior, or passage of time. J. Neurosci. Res. 2018;96(4):671–687. doi: 10.1002/jnr.24115
- Demertzi A, Antonopoulos G, Heine L, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138(9):2619–2631. doi: 10.1093/brain/awv169
- Lutkenhoff E, Chiang J, Tshibanda L, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann. Neurol. 2015;78(1):68–76. doi: 10.1002/ana.24423
- Guldenmund P, Soddu A, Baquero K, et al. Structural brain injury in patients with disorders of consciousness: A voxel-based morphometry study. Brain Inj. 2016;30(3):343–352. doi: 10.3109/02699052.2015.1118765
- Annen J, Frasso G, Crone J, et al. Regional brain volumetry and brain function in severely brain-injured patients. Ann. Neurol. 2018;83(4):842–853. doi: 10.1002/ana.25214
- Morozova SN, Kremneva EI, Sergeev DV, et al. Conventional Structural Magnetic Resonance Imaging in Differentiating Chronic Disorders of Consciousness. Brain Sci. 2018;8(8):144–155. doi: 10.3390/brainsci8080144
- Legostaeva LA, Mochalova EG, Suponeva NA, et al. Difficulties in evaluation of chronic disorders of consciousness: approaches to clinical assessment and instrumental studies. Russian Journal of Anesthesiology and Reanimatology. 2017;62(6):449–456. EDN: YPLNJY doi: 10.18821/0201-7563-2017-62-6-449-456
- Solovyeva PI, Sinkin МV, Talypov АE, et al. Clinical assessment of patients with chronic disorders of consciousness by different medical specialists. Annals of Clinical and Experimental Neurology. 2022;16(2):44–49. doi: 10.54101/ACEN.2022.2.5
- Medina JP, Nigri A, Stanziano M, et al. Resting-State fMRI in Chronic Patients with Disorders of Consciousness: The Role of Lower-Order Networks for Clinical Assessment. Brain Sci. 2022;12(3):355–374. doi: 10.3390/brainsci12030355
- Rohaut B, Doyle KW, Reynolds AS, et al. Deep structural brain lesions associated with consciousness impairment early after hemorrhagic stroke. Sci Rep. 2019;9(1):4174. doi: 10.1038/s41598-019-41042-2
- Alnagger N, Cardone P, Martial C, et al. The current and future contribution of neuroimaging to the understanding of disorders of consciousness. Presse Med. 2023;52(2):104163. doi: 10.1016/j.lpm.2022.104163
- Bakulin IS, Kremneva EI, Kuznetsov AV, et al. Chronic disorders of consciousness. Piradov MA, editor. Moscow: Hot line — Telecom; 2020.
Supplementary files