Conventional and innovative imaging modalities in bladder cancer: Techniques and applications
- Authors: Masino F.1, Eusebi L.2, Muscatella G.1, Montatore M.1, Sortino G.2, Giannubilo W.3, Guglielmi G.1,4,5
-
Affiliations:
- Foggia University School of Medicine
- Carlo Urbani Hospital
- Civitanova Marche Hospital
- Dimiccoli Hospital
- IRCCS Casa Sollievo della Sofferenza Hospital
- Issue: Vol 5, No 2 (2024)
- Pages: 318-333
- Section: Reviews
- Submitted: 27.11.2023
- Accepted: 06.02.2024
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/623889
- DOI: https://doi.org/10.17816/DD623889
- ID: 623889
Cite item
Abstract
This narrative review describes the current status of imaging in the evaluation of bladder cancer, considering conventional technologies such as ultrasonography, computed tomography urography, and magnetic resonance imaging, as well as novel technologies such as contrast-enhanced ultrasonography and dual-energy computed tomography.
The article is organized by first presenting an introduction on both the anatomy of the bladder (to understand its normal appearance on imaging) and the main features of bladder cancer with reference to epidemiology, clinical picture, classification, and treatment. Subsequently, the role of imaging is discussed, with an explanation of the technique and applications in bladder cancer assessment for each modality.
Imaging plays a critical role in the detection and staging of bladder cancer. In particular, the role of magnetic resonance imaging is expanding because it enables differentiating muscle-invasive bladder cancer from non-muscle-invasive bladder cancer using the Vesical Imaging-Reporting and Data System (VI-RADS), along with conventional technologies, such as computed tomography urography and ultrasonography. Contrast-enhanced ultrasound and dual-energy computed tomography are new imaging modalities that offer special advantages and provide the right approach to patients with oncological conditions. This review ends with the presentation of integrated imaging modalities such as positron emission tomography combined with computed tomography or magnetic resonance imaging, which are promising methods for bladder cancer staging.
Full Text
INTRODUCTION BLADDER ANATOMY
The urinary bladder is a subperitoneal, hollow muscular sac that serves as the reservoir of urine and allows its expulsion. This highly deformable organ is located in the pelvic cavity, behind the symphysis pubis, and below the parietal peritoneum. The size and shape of the urinary bladder change depending on how much urine it holds, with a total storage volume of up to 500 mL, and the pressure exerted by other organs. In particular, when filled, the bladder acquires a round or oval form [1].
The bladder’s anatomical structure is complex. Macroscopically, it is divided into four parts: the apex or dome, which is directed anterosuperiorly; the body; the fundus; and the neck, which is positioned inferiorly. The bladder’s floor has three openings, creating the trigone. The base of the trigone is formed by two openings from the ureters, which come from a short, oblique, intramuscular course. The third is the urethral opening, located at the bladder neck, precisely at the inferior angle of the trigone, where urine is expelled from the bladder [2, 3].
Microscopically, the bladder has a layered composition, similar to that of the ureters. The inner lining is made up of a transitional epithelium, known as the urothelium, which consists of transitional cells. In a relaxed state, the urothelium is 5–7 layers thick; however, it can stretch to accommodate increased urine volume. The lamina propria or submucosa, which is a subepithelial connective tissue containing muscle fibers with variable disposition, lies beneath the urothelium. Then, the muscularis propria is composed of the detrusor muscle, featuring the inner longitudinal, middle circular, and outer longitudinal layers. This smooth muscle is responsible for contracting and releasing urine from the bladder. Bladder walls are covered by serosa, a thin connective tissue layer that continues with the peritoneal layer of the abdominal wall and contains blood vessels. In areas without serosa, the bladder is enveloped by the adventitia, a layer of loose connective tissue [4, 5].
BLADDER CANCER
Bladder cancer (BCa) is a prevalent and aggressive malignancy worldwide, secondary to prostate cancer considering urogenital tumors [6]. The International Agency for Research on Cancer has documented that the following risk factors are associated with BC: tobacco smoking; certain occupational exposures, such as working in industries like aluminum production, rubber production, painting, firefighting, and exposure to various dyes (e.g., magenta and auramine) or dye intermediates (e.g., 4-aminobiphenyl); environmental factors such as X-ray radiation, gamma radiation, and arsenic; specific medications such as cyclophosphamide; opium consumption; and Schistosoma infection. Other risk factors, such as dietary elements, microbiome imbalances, gene–environment interactions, exposure to diesel exhaust emissions, and pelvic radiotherapy, have shown correlations with BCa development [7].
The most common presentation symptom of BCa is an asymptomatic macro or microhematuria, known as “painless hematuria,” which occurs in approximately 85% of patients. To identify the origin of the bleeding, hematuria must be carefully characterized as initial, terminal, and total [8]. Other frequent presenting symptoms of BCa are linked to bladder irritability, such as urinary frequency, urgency, and dysuria. Flank pain appears when ureteral obstruction occurs. Less common symptoms are lower extremity edema and palpable pelvic masses. In advanced cases, patients present with weight loss and abdominal or bone pain because of distant metastases [9, 10].
Urothelial carcinoma (UC) is the most common BCa subtype, followed by squamous cell carcinoma, sarcoma, lymphoma, and adenocarcinoma. Two-thirds of all BCa cases are non-muscle-invasive BC (NMIBC), whereas one-third are muscle-invasive BC (MIBC) and are related to a higher risk for metastasis and a significantly worse prognosis [7]. BCa morphology can vary depending on tumor growth and progression. For example, horizontal growth is typical of carcinoma in situ (CIS), whereas exophytic polypoid masses or sessile infiltrative lesions are typical of invasive forms [8, 6].
BCa is staged using the standard TNM system; as with other hollow organs, the T parameter is based on the depth of invasion of the layers. In particular, pTa refers to papillary carcinoma, an NMIBC type that presents as an exophytic mass lesion, whereas pTis refers to the flat CIS, included in NMIBC. At T1 stage, the tumor invades the lamina propria and is typically treated with transurethral resection of the bladder tumor (TURBT) and adjuvant intravesical therapy. At T2, the tumor invades the detrusor muscle, becoming an MIBC. T3 occurs when a tumor infiltrates the perivesical fat, and when it affects the surrounding organs, T4 commences. Tumors at T2 and above require more aggressive management, such as radical cystectomy [11]. The N parameter considers the lymph node involvement. N1 and N2 require the presence in the true pelvis of one or multiple nodes, respectively. In N3, the metastasis reaches the common iliac node. M1 indicates the presence of metastasis, with subclassifications of M1a when there is no regional lymph node involvement and M1b when other distant metastases are present [5, 12].
IMAGING MODALITIES
Imaging modalities, including ultrasonography (US), computed tomography urography (CTU), and magnetic resonance imaging (MRI), play an important role in diagnosing and staging BCa. They are crucial for BCa detection and differentiating a T1 from a T2, considering that the treatment changes significantly between the two stages [13].
Considering the sensitivity of BCa detection, this parameter increases from US to CTU, reaching a very high rate with MRI. Nevertheless, the latter is gaining wider applications because it is essential in differentiating an NMIBC from an MIBC.
International guidelines recommend US, not excluding physical examination, as the first step in the diagnostic workup of a suspicious tumor, as in the case of painless hematuria, and requires cystoscopy and subsequent biopsy for the final diagnosis [8].
Radionuclide diagnostics such as positron emission tomography (PET)/CT and PET/MRI have been discussed because hybrid imaging methods appear to be promising imaging tools in BCa staging, particularly for the detection of lymph node and distant metastases, as they are more accurate than conventional CT.
This narrative review also includes imaging modalities such as dual-energy CT (DECT) and contrast-enhanced US (CEUS) that are not mentioned in the habitual diagnostic workup but have utility in particular applications.
ULTRASONOGRAPHY
Technique
The patient should drink 300–500 mL of water before the examination to enable adequate distension of the bladder, which should be filled moderately. If underdistended, the evaluation of the bladder wall is limited because a wall thickening or a focal mass can be overestimated, whereas overdistention leads to patient discomfort and low cooperation.
Transabdominal US is mostly performed, which is analyzed in this review. Transvaginal US is performed in women to improve spatial resolution if needed, whereas in men, transrectal US can be performed if the transabdominal approach is limited. The examination is performed with the patient in the supine position, with lateral decubitus when required.
A convex probe (4.5–6 MHz) is more appropriate, and an abdomen/renal preset is suggested. For correct evaluation of the organ, the probe should be placed just above the symphysis pubis and angled caudally, and scanning should be performed in two orthogonal planes and in the oblique direction. In this way, the bladder is always centered within the field of view during the examination.
The bladder wall appears as layered with the hypoechoic muscle between two hyperechoic layers corresponding to the serosa and mucosa. The lateral and posterior walls are well visualized in US, and the anterior wall is affected by the reverberation phenomena, which can be adjusted with the time–gain compensation. To selectively explore the anterior wall and the vesical cupola, the examination can be performed using a higher-frequency linear transducer (>7.5 MHz). To evaluate the ureteral jet, which is a normal and periodic efflux of urine from the ureter into the bladder, color Doppler is necessary for the trigone of the posterior wall to exclude a complete ureteral obstruction.
US allows for the evaluation of the vesical capacity and residual urine volume. The urine volume is estimated by taking the three dimensions in the two orthogonal planes and applying an automated formula that includes a correction factor (k) that considers the complex shape of the bladder [11, 14].
Applications
Transabdominal US is influenced by various factors, such as the amount of vesical filling, the patient’s constitution, tumor size and distribution, or previous treatments (radiotherapeutic, chemotherapeutic, or surgical) [13]. US is reported to be 63% sensitive; in particular, the sensitivity rate decreases when male patients suffer from prostate hypertrophy, which indicates irregularity of the bladder base wall. On the contrary, the sensitivity rate increases when compared with cystoscopy in the case of a tumor within the diverticula because evaluation of the narrow neck by cystoscopy is limited [14].
Generally, BCa can be easily detected when is localized on the lateral and posterior walls, considering that most UC cases are localized in the posterior walls, and larger, which is the most important factor that influences the diagnostic sensitivity of US. Tumors can only be detected if their maximum diameter is >5 mm. A larger tumor is often associated with other signs such as wall rigidity and asymmetrical bladder distension. The site of origin of the tumor is a less important factor that influences sensitivity, although some regions (dome, anterior wall, and base) are more difficult to evaluate because of technical reasons [11].
Bladder masses are commonly echogenic, and shaped irregularly such as cauliflower-like, and are found either mounted on the bladder wall or in areas of the bladder wall with irregularly increased thickness. However, BCa features in US can differ depending on morphology and appear as papillary, infiltrating, or invasive, as well as with mixed features of papillary and infiltrating. Papillary forms appear as small echogenic masses originating from the bladder wall and projecting into the lumen, which are easily detected when larger than 2–3 mm. Conversely, if the tumor is a superficial carcinoma, it can be recognized only based on a soft wall thickening, which presents a normal echo structure and is not a sign of invasion. Infiltrating tumors have typical small papillary components and hypoechogenicity compared with the echogenicity of the vesical wall and the perivesical adipose tissue [11, 13, 14].
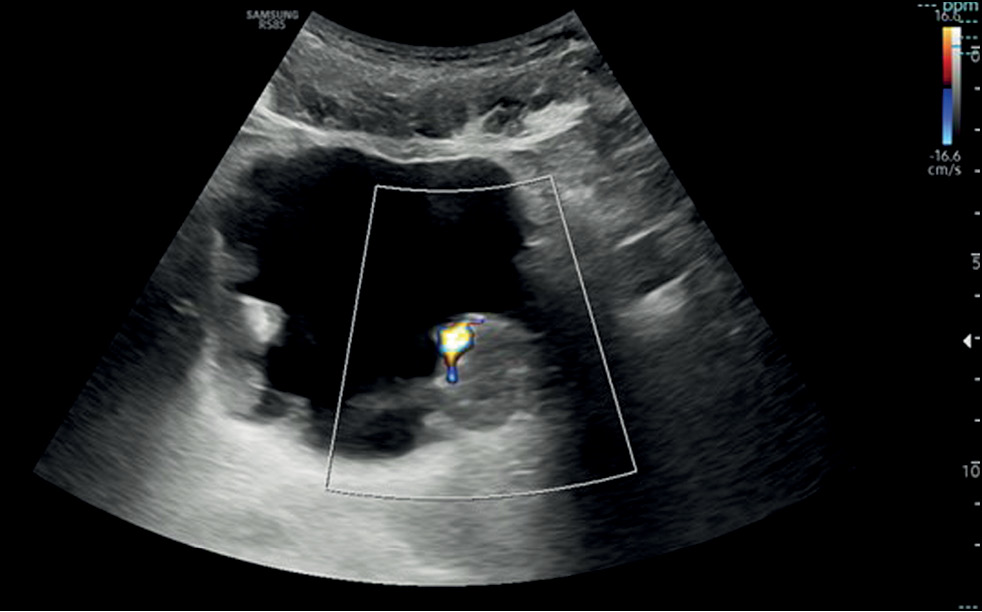
If a focal mass is detected in the US image, the presence of additional lesions must be further explored, considering that one-third of tumors are multifocal, and an additional evaluation with Doppler that helps in identifying the internal vascularity with a rich blood flow signal or a stellate morphology and differentiating a potential tumor from a blood clot. The latter can be excluded by asking the patient to change position from supine to lateral to assess for lesion mobility typical of a clot or performing bladder irrigation, followed by another US scan [11] (Fig.1).
Fig. 1. Transverse ultrasound image of the bladder showing diffuse irregular wall thickening with multiple masses and endoluminal development. Color Doppler on the largest echogenic lesion, localized on the left posterior bladder wall, showed vascularity within the mass.
CONTRAST-ENHANCED ULTRASONOGRAPHY
Technique
CEUS, a novel technology, can objectively reflect tissue perfusion, using ultrasound contrast agent (UCA). In conventional US, the bladder should be adequately distended before the examination. Initially, a complete baseline US evaluation should be performed before CEUS. The most commonly used UCA is sulfur hexafluoride (SonoVue), which is a blood pool tracer that never leaves the blood vessel and can be used for real-time dynamic imaging of microcirculation perfusion. It is injected intravenously (IV) in an amount of 2.4 mL, using a 21-G peripheral cannula, followed by approximately 5 mL of saline. The UCA remains in the circulation for a period sufficient to reach the organ and guarantee adequate interpretation of both arterial and venous phases. The study is conducted in basal B-mode conditions with a low mechanical index to reduce the incidence of microbubble rupture.
A normally distended bladder has a thin wall of approximately 2 mm, with little signal to the CD, and is nearly imperceptible in the initial phase of administration of the contrast medium, with a progressive signal enhancement up to approximately 2 min [15].
Applications
CEUS utilizes the biological principle that tumors exhibit distinct neovascularization patterns, leading to variations in contrast agent wash-in and washout times compared with non-neoplastic conditions. A key advantage of CEUS is its real-time capability. Unlike CT or MRI, which requires determining the optimal acquisition time for better differentiation of tumors from the surrounding bladder wall, CEUS does not necessitate such precise timing because the enhancement pattern can vary among patients due to factors such as cardiovascular health or the extent of microvascularization in BCa. With CEUS, a dynamic real-time assessment of enhancement can be performed continuously, eliminating the need to pinpoint a specific moment.
Moreover, prolonged BCa enhancement allows for a comprehensive exploration of bladder walls with just one dose of contrast agent, making it useful in detecting multiple cancer foci in multicentric cancers. With arterial neovascularization, a common feature of BCa, the signal enhancement in papillary and sessile lesions or small focal thickening areas is immediately noticeable, similar to that in the arterial phase of uro-CT. The signal increase is typically uniform, except in larger, high-grade, invasive cases where it may be non-uniform, particularly in necrotic regions within the tumor [16].
After the rapid arterial phase, most tumors reach a plateau with slow washout, although the venous phase can vary based on size and cellular differentiation. CEUS is valuable for differential diagnosis, helping distinguish neoplastic growths from other bladder wall alterations that may mimic tumors, such as intravesical clots, adherent lithiasis, benign prostatic hypertrophy-related thickening, or inflammation-induced wall thickening. Focal or nodular enhancement indicates neoplasia in these situations.
BCa detection with CEUS relies on identifying areas of focal hyperenhanced wall thickening or enhancing masses protruding into the bladder lumen. The use of a contrast agent in US improves BCa detection, particularly in cases where traditional US studies may be inconclusive because of factors such as inadequate bladder distension, history of bladder surgeries, obesity, or the presence of an intravesical catheter.
The depth of wall invasion, histological grade, and extension beyond the bladder are the main factors for determining the prognosis and treatment approach for BCa. Although MRI and CT are the preferred modalities for local staging, CEUS can aid in evaluating wall invasion by assessing the enhancement pattern of the bladder wall. It can help differentiate a noninvasive UC from an infiltrating carcinoma based on the presence or absence of a hypoechoic layer and the enhancement pattern after arterial enhancement [15].
Malignant bladder tumors exhibit distinct enhancement patterns compared with benign lesions, making CEUS a valuable tool for distinguishing between them and improving diagnostic accuracy. CEUS enables real-time observation of the blood flow in bladder tumors, aiding in the differentiation of benign from malignant tumors. However, compared with CT and MRI, its usefulness in bladder staging for infiltrating carcinomas is limited because it cannot assess perivesical fat infiltration and retroperitoneal lymph nodes (Fig. 2) [16].
Fig. 2. Sagittal contrast-enhanced ultrasound images showing an enhancing mass of the left bladder wall, not well definable in the B-mode as noticed on the left side of the image.
COMPUTED TOMOGRAPHY UROGRAPHY
Technique
CTU, a CT examination of the urinary tract, is performed with an unenhanced scan and after IV contrast material administration with a multiphasic acquisition to obtain a set of images that show a fully opacified and distended intrarenal collecting system, ureters, and bladder [17].
More precisely, the protocol includes an unenhanced scan of the abdomen and pelvis. After IV administration of contrast agent, the phases obtained are as follows: a corticomedullary phase 30–40 sec after the injection, resulting in an arterial phase; a nephrographic phase 100 sec after the injection; and an excretory phase 8–12 min after the injection. The scan should be in a craniocaudal direction, and the extension on the Z-axes should start from the diaphragmatic dome to reach the pubic symphysis, particularly in the unenhanced and nephrographic scan, whereas the corticomedullary and excretory phases can start from the upper pole of the kidney.
However, the main limitation of a multiphasic protocol is the high radiation exposure, ranging from 25 to 35 mSv. For this reason, particularly in young patients, a split-bolus technique is suggested. This includes a two-phase protocol with an unenhanced scan, followed by two IV injections of contrast agent of approximately 80 and 40 mL. After the first administration, the corticomedullary scan is obtained after 20 sec. After an 8-min delay, the second bolus is administered, followed by a scan at 100 sec to obtain the nephrographic–excretory phase. The scan should be in a craniocaudal direction, and the extension on the Z-axes should start from the diaphragmatic dome to reach the pubic symphysis, particularly in the unenhanced and nephrographic–excretory scan, whereas the corticomedullary phase can start from the upper pole of the kidney [18].
Both protocols may be completed with an IV administration of 10 mg of furosemide 2–3 min before the corticomedullary scan to obtain adequate distension of the upper urinary tracts and the bladder. Therefore, an underdistended bladder can appear thickened, particularly along its anterior wall, and the lumen can show an incomplete mixing of nonopacified urine and contrast material, resulting in a urine contrast level because the specific gravity of the contrast medium is higher than that of urine [17].
Applications
Abdominal CTU is the most commonly used technique, thanks to its many advantages, such as wide availability, fast scanning, and creation of multiplanar reformatted and three-dimensional (3d) reconstructed images. In patients suspected or diagnosed with BCa, the examination is performed for BCa detection and staging, in the latter case to assess the locoregional and distant extension of the disease.
Each phase of the protocol required in a CTU has advantages. The unenhanced CT scan is used to measure the basal attenuation of the mass to compare it after contrast enhancement and identify the presence of stones, calcifications, hemorrhages, and clots. The corticomedullary phase is used to evaluate suspected vascular abnormalities or arterial enhancements. The nephrographic phase is used to detect and characterize renal masses. The excretory phase is used to assess the urothelium because the bladder is filled with dense contrast material and an endoluminal soft tissue lesion will appear as a filling defect [19].
BCa can appear as a focal region of bladder wall thickening or as a mass protruding into the bladder lumen or extending into adjacent tissues in advanced cases. If the bladder distension is not adequate, the asymmetry of the thickening must be examined. Generally, masses have soft tissue attenuation and may be encrusted with small calcifications.
CTU has the highest accuracy, with a pooled sensitivity of 92% and a pooled specificity of 95% in detection and staging. Concerning T stages, it is limited in differentiating NMIBC from MIBC but can distinguish T3 and T4 tumors. Regarding N stages, it enables the assessment of lymph node morphology and size. As regards the size, a suspect is made when the pelvic, abdominal, and retroperitoneal lymph nodes have a short axis, greater than 8 and 10 mm. Regarding the morphological criterion, the presence of confluent lymph nodes or those with a necrotic center is considered a clear sign of lymph node metastasis. In the study of lymph nodes, CTU is limited by potential overstaging, detected in approximately 30% of cases with reactive lymphadenopathies, with a short axis >10 mm, and potential substaging when lymph nodes are malignant but have dimensions within the limits. Concerning M stages, BCa most frequently metastasizes to the pelvic and retroperitoneal lymph nodes. The bone is the most common site for distant BCa metastases; most appear sclerotic but can also be lytic or mixed lytic sclerotic. In solid organs, the liver and lung are the most frequent sites of metastasis, and other organs are far less frequently involved [11].
After the diagnostic workup, for detection requiring further examination, an endoscopy with biopsy may be indicated to confirm the diagnosis and determine the number, extent, and localization of the urothelial tumors [18].
DUAL-ENERGY COMPUTED TOMOGRAPHY
Technique
DECT is a novel imaging technology that operates two X-ray tubes with different kilovoltages (one lower and one higher) to images reconstructed in post-processing.
Considering the purpose of the examination such as the detection of a suspicious BCa or its staging, the most useful types of images are the virtual monochromatic (VMC), virtual non-contrast (VNC), iodine map, and atomic map.
VMC generates images similar to those of conventional single-energy CT considering quality; however, it provides more reliable attenuation values. The lower-energy kilovoltage setting can increase contrast among near structures, thanks to the high beam attenuation of iodine. Consequently, a parietal lesion is easier to recognize. The higher-energy kilovoltage setting can decrease noise and artifacts. The comparison between the two different kilovoltages settings, from VMC-acquired images, also produces a spectral attenuation curve, which is a function of energies. The latter is attributed to its properties, which are useful to improve lesion characterization.
VNC generates images “without contrast” by suppressing the iodine material uptake from scans acquired post-contrast. Therefore, VNC images are also known as iodine-removed images. Accordingly, the radiation dose could be reduced because the patient has not undergone the first unenhanced scan.
The iodine map is a material-specific image in opposition to the iodine-removed image, as iodine is selected and not suppressed to show all areas with iodine uptake. This image results in a color map that can quantify the iodine uptake expressing it in mg/mL. Moreover, it allows for distinguishing a vascularized lesion from a nonvascularized lesion considering the amount of iodine filling the aorta.
The effective atomic number map is a quantitative method for assessing material differentiation and evaluating attenuation variations as a function of energy [3].
Applications
DECT, a new imaging method, may help overcome the main limitations of CT such as ionizing radiation overexposure typical of patients with oncological conditions who underwent repeated acquisitions and tight follow-up.
Moreover, DECT allows for better lesion characterization, thanks to post-processing reconstruction.
VNC images provide a true unenhanced image that helps exclude the presence of stones, calcifications, and fresh bleeding that appears hyperdense in the typical basal scan and in measuring the attenuation value of reference for the subsequent post-contrast graphic scans.
The spectral curve, in the case of bladder wall thickening, shows a curve tending to increase from lower values of kilovoltage setting.
VMC images at low-energy kilovoltage settings generate better contrast of the tumor despite the nearby regions and increase the sensitivity in tumor detection. Moreover, by normalizing the iodine quantification to that of the aorta, in the nephrographic phase, this image type had increased specificity when a threshold of ≥3.0 mg/mL is reached and allows the differentiation of a vascular from a nonvascular lesion. The formula is as follows: |I| normalized=|I| lesion⁄|I| aorta [20, 21].
DECT advantages also concern BCa staging because iodine maps enable easier evaluation of the tumor infiltration of wall layers, including the muscular layer in differentiating an NMIBC from an MIBC and evaluating lymph node involvement and presence of metastases.
For treatment planning, the application of this technology may be crucial because it can better assess the relationship between the tumor and a vascular structure, with increased contrast obtained with the VMC at a lower kilovoltage setting, offering an important parameter (Fig. 3 and 4) [22].
Fig. 3. Multiplanar iodine map images showing different attenuations of multifocal masses, the main localized on the left posterior wall, with different Av values compared with the Av endoluminal value. The spectral curve (upper left side) allowed the characterization of materials because each material has a different attenuation curve.
Fig. 4. Multiplanar iodine map with coloring overlap showing different attenuations of multifocal masses, the main localized on the left posterior wall, with different Av values compared with the endoluminal Av value. The spectral curve (upper left side) allowed the characterization of materials because each material has a different attenuation curve.
MAGNETIC RESONANCE IMAGING
Technique
For adequate examination, the preparation of the patient with moderate bladder distention is crucial. The patient should urinate and start drinking 500 mL of water about 2.5 h before MRI.
A targeted scan with the localizer can guide the technician in starting the test when the bladder is properly distended.
Bladder distension, as mentioned before, is essential in BCa evaluation. The bladder wall may appear thickened, and underdistension may lead to a misdiagnosis. Conversely, overdistension may cause discomfort in patients, who would move during artifact detection, or interrupt the examination if the patient could not hold more urine.
Generally, using a 1.5-T MRI scanner, the examination is performed with the patient in a supine position, and sequences necessary for a proper bladder evaluation are as follows: T1-weighted (T1W) fast spin echo on the axial plane; T2W sequences with high resolution and narrow field of view on axial, sagittal, or coronal plane and with fat suppression; diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC); and finally, DCE-MRI with T1W 3D gradient echo and the Dixon three-point method.
In female patients, images must include not only the urinary bladder but also the uterus, ovaries, and vagina, whereas in male patients, the images must include the prostate [5].
Applications
In BCa evaluation, MRI is mainly applied in local tumor staging because it allows distinguishing the presence and absence of muscular infiltration, resulting in the differentiation between NMIBC and MIBC, and stages ranging from T1 to >T2.
The bladder wall has multilayers, with the urothelium and lamina propria appearing as a hyperintense line only after contrast agent administration, in the early phase of DCE-MRI sequences. The muscular layer appears as a low-intensity line on T2W, medium-intensity line in DWI and ADC sequences, and with a late and gradual enhancement in DCE-MRI [5].
The development of the vesical imaging-reporting and data system (VI-RADS) score helps standardize the approach to MRI acquisition, interpretation, and reporting in patients diagnosed with BCa through TURBT. The score ranges from 1 to 5 and expresses the increasing risk of invasion of the detrusor muscle [6]. For accurate examination, the sequences include T2W, DWI/ADC, and DCE with each sequence generating a score of 1–5. T1W is not useful for differentiating MIBC from NMIBC because the detrusor muscle shows intermediate-signal intensity as well as a cancerous process [23].
Initially, the structural information in the T2W must be analyzed, evaluating the integrity of the muscular layer in T2W that should appear as homogeneously hypointense in contrast with a hyperintense signal of the bladder content. Then, the signal on DWI/ADC and DCE sequences must be evaluated. In tumors, the signal would appear hyperintense on DWI and hypointense on the ADC map, and there is an early enhancement of the inner layer. After obtaining information from each sequence, the combination of the different scores is compared to obtain the final VI-RADS score.
VI-RADS 1 is assigned when there is an interruption of the intensity signal line corresponding to the muscular layer in T2W. The maximum size reached from the lesion (sessile or vegetating) is 1 cm. VI-RADS 1 suggests an NMIBC.
VI-RADS 2 is assigned when there is an interruption of the intensity signal line but with a diameter >1 cm. The lesion could be associated with edema, appearing with a thickening line, and related to an increasing probability of invasion. VI-RADS 3 expresses a doubt: there is no clear disruption of the low-signal intensity of the muscular layer in T2W. VI-RADS 4 is assigned when there is a certain invasion of the muscular layer. VI-RADS 5 is assigned when the muscular layer invasion is associated with the involvement of the nearly adipose tissue.
In case of a discrepancy in results, the DWI/ADC map and DCE will prevail to downgrade and upgrade lesions [5].
MRI also plays a role in post-therapeutic approach in BCa, concerning patient evaluation after neoadjuvant chemotherapy and immunotherapy, which is the last revolution in the treatment MIBC. The purpose is to assess the lesion after treatment under T2W, DWI/ADC, and DCE sequences and establish the response to the therapy, which can be partial, complete, or absent. In this context, the VI-RADS scoring system has shown promising results [24].
In conclusion, MRI is rapidly becoming a leading imaging modality in BCa diagnostic workup, assessment of response to therapies, and longitudinal surveillance and plays an important role in treatment planning for BCa surgical and radiation therapy. Nevertheless, transurethral resection biopsy is required for tumor grading and cannot be replaced by MRI (Fig. 5 and 6).
Fig. 5. (a) Multiplanar T2W sequences showing a mass on the left posterior wall, >1 cm in size, with an intermediate signal of the muscular layer (VI-RADS 4). (b) DWI sequence and ADC maps showing a lesion with significantly limited diffusion, extending through the muscular layer. The low ADC value of approximately 0.9 × 10−3 mm2/sec denote malignancy (VI-RADS 4). (c, d) DCE sequence showing early and heterogeneous enhancement of the lesion, extending through the muscular layer (VI-RADS 4). The VI-RADS overall score was four. Image source: Eusebi Laura, Masino Federica, Gifuni Rossella, Fierro Davide, Michele Bertolotto, Cova Maria Assunta, Giuseppe Guglielmi. Role of Multiparametric-MRI in Bladder Cancer. Current Radiology Reports 11, 69–80 (2023). https://doi.org/10.1007/s40134-023-00412-5. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license 4.0 (http://creativecommons.org/licenses/by/4.0/).
Fig. 6. (a) Multiplanar T2W sequences showing a mass, >1 cm, on the right lateral wall of the bladder dome, with an intermediate signal extending through the muscular layer and invading the perivesical adipose tissue (VI-RADS 5). (b) DWI sequence and c ADC map showing a significantly limited diffusion lesion extending through the muscular layer and invading the perivesical adipose tissue (VI-RADS 5). (c) DCE showing an early and heterogeneous improvement of the lesion extending through the muscular layer and the perivesical adipose tissue (VI-RADS 5). The VI-RADS overall score was five. Image source: Eusebi Laura, Masino Federica, Gifuni Rossella, Fierro Davide, Michele Bertolotto, Cova Maria Assunta, Giuseppe Guglielmi. Role of Multiparametric-MRI in Bladder Cancer. Current Radiology Reports 11, 69–80 (2023). https://doi.org/10.1007/s40134-023-00412-5. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license 4.0 (http://creativecommons.org/licenses/by/4.0/).
RADIONUCLIDE HYBRID IMAGING: POSITRON EMISSION TOMOGRAPHY/COMPUTED TOMOGRAPHY and POSITRON EMISSION TOMOGRAPHY/MAGNETIC RESONANCE IMAGING
PET/CT combines PET and CT into a single imaging modality. 2-Fluorine-18-fluoro-2-deoxy-d-glucose (FDG) is the most common radiotracer in oncology; therefore, FDG PET/CT is widely used in the clinical management in many cancer types [25, 26].
As an analog of glucose, 18F-FDG is taken up within tumor cells via GLUT and other transporters where it is phosphorylated by hexokinase but not further metabolized, leading to intracellular accumulation. PET/CT offers a high-sensitivity scan for metabolic activity with precise anatomical localization [27].
In BCa, in recent years, this hybrid imaging technique is increasingly used for recurrence detection after radical cystectomy [25, 26].
Considering that urothelial tumors have a high FDG uptake, urinary excretion of FDG may mask tumors in all the urinary tract extension, particularly in the bladder. Because of this important limitation in this technique, several methods have been investigated and tested to reduce urine FDG activities, particularly profuse water uptake, diuretic administration, and catheterization are helpful. However, catheterization that involves flushing and retrograde bladder filling can increase the risk of iatrogenic urinary tract infection and consequently increase hospitalization times. Conversely, this limitation consists in the application of early dynamic images that could be useful for BCa detection before the excretion and collection of FDG in the bladder [25].
Currently, FDG PET/CT is not recommended as the initial diagnostic or as a primary staging tool because it is assumed to be unable to evaluate microscopic perivesical fat invasion and adjacent-organ involvement. Nevertheless, it may be used to assess treatment response, detect any residual or recurrent diseases, and differentiate scar tissue from active tumors [26].
PET/MRI is a hybrid imaging modality that combines the functional information provided by PET and the detailed anatomical images obtained through MRI. The PET component, typically using FDG as a tracer, highlights areas with increased metabolic activity, which is often indicative of cancerous tissue. Conversely, the MRI component provides a great contrast of soft tissue. Considering these premises, PET/MRI could overcome the intrinsic limitation of PET/CT in assessing local disease extent because quality MR images can help in assessing the spread of malignant tissue in the perivesical fat and the involvement of the muscular layer [28]. Nevertheless, the sensitivity of PET/MRI is still very low in diagnosing early-stage BCa because of the renal excretion of the FDG PET tracer; thus, small lesions in the bladder wall can be missed [29].
CONCLUSIONS
Imaging is crucial in BCa evaluation, particularly in detection and staging. Conventional technologies such as US, particularly CTU, are nowadays flanked by MRI, which is acquiring importance because it allows the differentiation of NMIBC from MIBC through VI-RADS. Combined imaging techniques such as PET/CT and PET/MRI are promising tools in BCa staging. Emerging modalities such as CEUS and DECT are not included in the typical diagnostic and staging process but have applications in particular conditions and can provide useful information for both the clinicians and radiologists to guarantee the proper approach to patients with oncological conditions, with a future eye on increasingly personalized medicine.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
About the authors
Federica Masino
Foggia University School of Medicine
Email: federicamasino@gmail.com
ORCID iD: 0009-0004-4289-3289
MD
Italy, FoggiaLaura Eusebi
Carlo Urbani Hospital
Email: lauraeu@virgilio.it
ORCID iD: 0000-0002-4172-5126
MD
Italy, JesiGianmichele Muscatella
Foggia University School of Medicine
Email: muscatella94@gmail.com
ORCID iD: 0009-0004-3535-5802
MD
Italy, FoggiaManuela Montatore
Foggia University School of Medicine
Email: manuela.montatore@unifg.it
ORCID iD: 0009-0002-1526-5047
MD
Italy, FoggiaGiuseppe Sortino
Carlo Urbani Hospital
Email: giuseppesortino@live.it
ORCID iD: 0000-0002-8804-1805
MD
Italy, JesiWilly Giannubilo
Civitanova Marche Hospital
Email: willygiannubilo@virgilio.it
MD
Italy, Civitanova MarcheGiuseppe Guglielmi
Foggia University School of Medicine; Dimiccoli Hospital; IRCCS Casa Sollievo della Sofferenza Hospital
Author for correspondence.
Email: giuseppe.guglielmi@unifg.it
ORCID iD: 0000-0002-4325-8330
Professor
Italy, Foggia; Barletta; San Giovanni RotondoReferences
- Hill WG. Control of Urinary Drainage and Voiding. Clin J Am Soc Nephrol. 2015;10(3):480–492. doi: 10.2215/CJN.04520413
- Glassock RJ, Rule AD. Aging and the Kidneys: Anatomy, Physiology and Consequences for Defining Chronic Kidney Disease. Nephron. 2016;134(1):25–29. doi: 10.1159/000445450
- Montatore M, Muscatella G, Eusebi L, et al. Current Status on New Technique and Protocol in Urinary Stone Disease. Curr Radiol Rep. 2023;11(12):1–16. doi: 10.1007/s40134-023-00420-5
- Sam P, Nassereddin A, LaGrange CA. Anatomy, Abdomen and Pelvis: Bladder Detrusor Muscle. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Eusebi L, Masino F, Gifuni R, et al. Role of Multiparametric-MRI in Bladder Cancer. Curr Radiol Rep. 2023;11(5):69–80. doi: 10.1007/s40134-023-00412-5
- Nicola R, Pecoraro M, Lucciola S, et al. VI-RADS score system — A primer for urologists. Int Braz J Urol. 2022;48(4):609–622. doi: 10.1590/s1677-5538.ibju.2021.0560
- Jubber I, Ong S, Bukavina L, et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur Urol. 2023;84(2):176–190. doi: 10.1016/j.eururo.2023.03.029
- Messina E, Pecoraro M, Pisciotti ML, et al. Seeing is Believing: State of the Art Imaging of Bladder Cancer. Semin Radiat Oncol. 2023;33(1):12–20. doi: 10.1016/j.semradonc.2022.10.002
- Compérat E, Amin MB, Cathomas R, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 2022;400(10364):1712–1721. doi: 10.1016/S0140-6736(22)01188-6
- Ahmadi H, Duddalwar V, Daneshmand S. Diagnosis and Staging of Bladder Cancer. Hematol Oncol Clin North Am. 2021;35(3):531–541. doi: 10.1016/j.hoc.2021.02.004
- Wentland AL, Desser TS, Troxell ML, Kamaya A. Bladder cancer and its mimics: a sonographic pictorial review with CT/MR and histologic correlation. Abdom Radiol. 2019;44(12):3827–3842. doi: 10.1007/s00261-019-02276-w
- Wong VK, Ganeshan D, Jensen CT, Devine CE. Imaging and Management of Bladder Cancer. Cancers. 2021;13(6):1396. doi: 10.3390/cancers13061396
- Messina E, Pisciotti ML, Pecoraro M, et al. The use of MRI in urothelial carcinoma. Curr Opin Urol. 2022;32(5):536–544. doi: 10.1097/MOU.0000000000001011
- Schallom M, Prentice D, Sona C, et al. Accuracy of Measuring Bladder Volumes With Ultrasound and Bladder Scanning. Am J Crit Care. 2020;29(6):458–467. doi: 10.4037/ajcc2020741
- Ahmadi H, Duddalwar V, Daneshmand S. Diagnosis and Staging of Bladder Cancer. Hematol Oncol Clin North Am. 2021;35(3):531–541. doi: 10.1016/j.hoc.2021.02.004
- Liu Q, Gong H, Zhu H, Yuan C, Hu B. Contrast-Enhanced Ultrasound in the Bladder: Critical Features to Differentiate Occupied Lesions. Comput Math Methods Med. 2021;2021:1–5. doi: 10.1155/2021/1047948
- Fouladi DF, Shayesteh S, Fishman EK, Chu LC. Imaging of urinary bladder injury: the role of CT cystography. Emerg Radiol. 2020;27(1):87–95. doi: 10.1007/s10140-019-01739-3
- Renard-Penna R, Rocher L, Roy C, et al. Imaging protocols for CT urography: results of a consensus conference from the French Society of Genitourinary Imaging. Eur Radiology. 2020;30(3):1387–1396. doi: 10.1007/s00330-019-06529-6
- Abuhasanein S, Hansen C, Vojinovic D, et al. Computed tomography urography with corticomedullary phase can exclude urinary bladder cancer with high accuracy. BMC Urol. 2022;22(1):60. doi: 10.1186/s12894-022-01009-4
- Bicci E, Mastrorosato M, Danti G, et al. Dual-Energy CT applications in urinary tract cancers: an update. Tumori. 2023;109(2):148–156. doi: 10.1177/03008916221088883
- Parakh A, Lennartz S, An C, et al. Dual-Energy CT Images: Pearls and Pitfalls. RadioGraphics. 2021;41(1):98–119. doi: 10.1148/rg.2021200102
- Toia GV, Mileto A, Wang CL, Sahani DV. Quantitative dual-energy CT techniques in the abdomen. Abdom Radiol (NY). 2022;47(9):3003–3018. doi: 10.1007/s00261-021-03266-723
- Lai AL, Law YM. VI-RADS in bladder cancer: Overview, pearls and pitfalls. Eur J Radiol. 2023;160:110666. doi: 10.1016/j.ejrad.2022.110666
- Panebianco V, Pecoraro M, Del Giudice F, et al. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J Magn Reson Imaging. 2022;55(1):23–36. doi: 10.1002/jmri.27361
- Bouchelouche K. PET/CT in Bladder Cancer: An Update. Semin Nucl Med. 2022;52(4):475–485. doi: 10.1053/j.semnuclmed.2021.12.004
- Kim SK. Role of PET/CT in muscle-invasive bladder cancer. Transl Androl Urol. 2020;9(6):2908–2919. doi: 10.21037/tau.2020.03.31
- Omorphos NP, Ghose A, Hayes JDB, et al. The increasing indications of FDG-PET/CT in the staging and management of Invasive Bladder Cancer. Urol Oncol. 2022;40(10):434–441. doi: 10.1016/j.urolonc.2022.05.017
- Zhang-Yin J, Girard A, Marchal E, et al. PET Imaging in Bladder Cancer: An Update and Future Direction. Pharmaceuticals (Basel). 2023;16(4):606. doi: 10.3390/ph16040606
- Muin D, Laukhtina E, Hacker M, Shariat SF. PET in bladder cancer imaging. Curr Opin Urol. 2023;33(3):206–210. doi: 10.1097/MOU.0000000000001090
Supplementary files