COVID-19-related cardiac lesion: The questions of pathogenesis and diagnostics
- Авторлар: Filatova D.A.1, Mershina E.A.1,2, Sinitsyn V.E.1,2
-
Мекемелер:
- Lomonosov Moscow State University
- Medical Research and Education Center of Lomonosov Moscow State University
- Шығарылым: Том 4, № 2 (2023)
- Беттер: 156-169
- Бөлім: Systematic reviews
- ##submission.dateSubmitted##: 25.02.2023
- ##submission.dateAccepted##: 31.03.2023
- ##submission.datePublished##: 12.07.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/284706
- DOI: https://doi.org/10.17816/DD284706
- ID: 284706
Дәйексөз келтіру
Аннотация
Coronavirus infection is still a topic of interest in the medical community today. Among the heterogeneous clinical manifestations of this disease, lesions of cardiac structures often occur. They are mainly inflammatory in nature and can be acute or delayed. Aside from myocarditis, coronavirus infection can induce cardiac injuries, including acute coronary syndrome, thromboembolic events, heart failure, and heart rhythm disturbances. It is well known that the prognosis for patients with cardiac lesions significantly worsens; timely diagnosis and treatment initiation play an important role in preventing severe complications.
This review presents the most recent literature data on the pathogenesis of cardiac lesions in COVID-19 patients and discusses the rational diagnosis of this pathology using modern techniques, such as laboratory, functional imaging (cardiac magnetic resonance is the most important of these), and invasive ones. It is now established that diagnosing myocarditis caused by coronavirus infection differs fundamentally from diagnosing other types of myocarditis. Furthermore, the main aspects of inflammatory heart lesions associated with COVID-19 vaccination are discussed, as this complication occurs more frequently than is commonly believed. It is often used as a rationale for refusing vaccination; however, this decision may severely affect the individual and the population.
Толық мәтін
INTRODUCTION
The coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 was first detected in December 2019 and quickly spread worldwide. Its clinical manifestations include all influenza symptoms, such as cough, fever, fatigue, dyspnea, anosmia, ageusia, and pharyngodynia, which can progress to acute respiratory distress syndrome and multiple organ failure. Furthermore, acute injury to heart structures, primarily the myocardium, is also possible. Many studies have found that high troponin levels are associated with higher mortality in patients with COVID-19. The timely and accurate diagnosis of this condition is critical for patient survival; however, it remains a challenge.
This review aimed to critically summarize available data on COVID-19-associated myocarditis and investigate the main aspects of its pathogenesis and differential diagnosis.
The PubMed database was searched for relevant publications. The search terms were “COVID-19/SARS-CoV-2 and myocardit*” in the titles of publications (as indicated by adding [ti]). Only full-text articles were selected, such as meta-analyses, systematic reviews, and reviews; these conditions were determined via the appropriate filters. The literature on post-vaccine myocarditis was selected by adding “and vaccin*” to the above search terms, and other search parameters were the same as described above.
Following data collection, the initial sample for the literature review included 126 publications, of which 67 discussed vaccinations against COVID-19.
COVID-19-RELATED CARDIAC LESION
COVID-19 is caused by the SARS-CoV-2, which was discovered in late 2019 in the Chinese city of Wuhan and quickly spread worldwide, causing a pandemic.
Possible symptoms of COVID-19 include fever, cough, dyspnea, anosmia, ageusia, and pharyngodynia. The most severe complications include acute respiratory distress syndrome and multiple organ failure. Clinical manifestations also include acute injury to the structures of the heart, primarily the myocardium. Approximately 20% of hospitalized patients have high specific troponins [1], which do not always correlate with other signs of myocardial damage. A study of troponin I levels in patients with COVID-19 on the first day of hospitalization found elevations in 36% of cases. Even minor myocardial damage is associated with a significant increase in mortality [2]. However, many aspects of myocardial damage in COVID-19 remain unclear.
Pathogenesis and clinical manifestations
A thorough understanding of the pathogenesis of cardiac lesion is critical because it allows early initiation of treatment and prevention of severe consequences, including death.
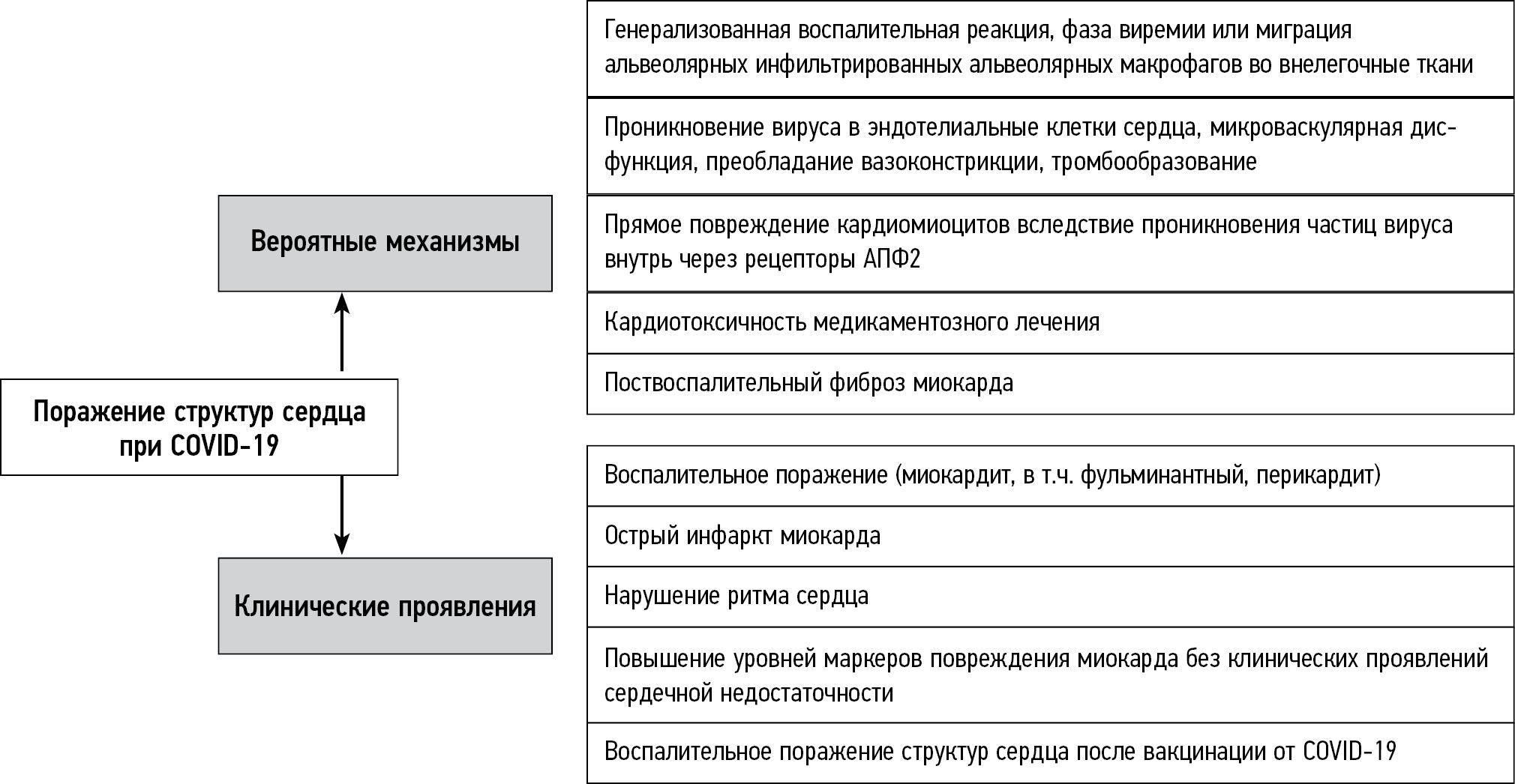
To date, little evidence shows that SARS-CoV-2 enters cardiomyocytes and causes direct harm to them. Associated lymphocytic myocarditis is common in patients with COVID-19, although its etiology is linked to a generalized cytokine-mediated inflammatory response. In several studies [3–5], myocardial biopsy revealed lymphocytic infiltration, interstitial edema, and isolated foci of necrosis; however, no intracellular viral material was found. Lindner et al. [3] discovered the COVID-19 pathogen in the myocardium while studying autopsy materials from patients with COVID-19 who did not have a clinical picture of fulminant myocarditis. However, it was found primarily in interstitial cells and macrophages that penetrated into the myocardium rather than in cardiomyocytes. The presence of the virus was not associated with increased mononuclear infiltration of the myocardium, and no histological signs of myocarditis were detected, i.e., no massive cellular infiltrates or necrosis areas were reported [6]. Other authors have reported timilar findings [7, 8]. According to Fox et al. [9], 10 autopsies of African Americans who died from COVID-19 revealed single-cell necrosis (without large areas of cardiomyocyte necrosis) in the myocardium without overt lymphocytic myocarditis. This suggested that viral particles were present in cardiac macrophages caused by a viremic phase or migration of infiltrated alveolar macrophages into extrapulmonary tissues. Moreover, the possible cardiotoxicity of drug therapy cannot be overlooked in the genesis of inflammatory myocardial damage identified at autopsy in patients with COVID-19 [10].
Another potential mechanism for cardiac lesion is direct virus entrance into the endothelial cells of the heart. The endothelium is a para-, auto-, or endocrine tissue, and its damage causes microvascular dysfunction and a shift in vascular homeostasis toward vasoconstriction, resulting in organ and tissue ischemia, inflammation, edema, and thrombosis. SARS-CoV-2 enters endothelial cells through angiotensin-converting enzyme 2 (ACE2) receptors, causing active inflammation. Some authors describe SARS-CoV-2-induced diffuse vasculitis: presumably, endothelitis can cause multiple organ damage typical of COVID-19 caused by microvascular dysfunction [11]; however, these data are currently limited and require additional confirmation.
Another theory is that cardiac lesion is triggered by an overactive immune system that releases various inflammatory mediators, and this condition is commonly described as “cytokine storm.” The activation of platelets, neutrophils, and other components of the inflammatory response results in micro- and macrovascular thrombosis, which leads to vascular occlusion and cell death. Both arterial and venous thrombosis is common in COVID-19 [12]. In an observational study, patients with COVID-19 and ST segment elevation myocardial infarction had higher troponin levels and higher incidence of thrombosis than patients with COVID-19 but without infarction [13]. Acute coronary syndrome without signs of coronary occlusion is also quite common. According to Bangalore et al. [14], only 44% of 18 patients with COVID-19 and ST segment elevation on the ECG were diagnosed with acute coronary thrombosis resulting in myocardial infarction, and non-coronary myocardial damage was observed in 56% of cases. Diagnostic quandaries are fairly common in these circumstances, particularly considering the nonspecific symptoms of myocarditis, which include fatigue, dyspnea, tachycardia, and chest tightness.
Inflammatory myocardial injuries seen in COVID-19 include fulminant myocarditis, which causes a rapid decrease in the left ventricular contractility, most often secondary to bilateral lung damage, and pericardial effusion leading to cardiac tamponade. Cardiogenic shock affects roughly one-third of patients, and the mortality rate is approximately 26%. Isolated cases of fulminant myocarditis following vaccination against COVID-19 have been reported; however, the disease has a less severe course. A study reported that the risk of fulminant myocarditis is influenced not by COVID-19 or vaccination but by a predisposition [15].
Rhythm disturbance is one of the most serious clinical manifestations of cardiac lesion. Although the true prevalence of this condition is unknown, evidence shows that arrhythmia results in a transfer to the intensive care unit in 44.4% of cases [16]. Moreover, determining how many COVID-19 arrhythmias are caused by electrolyte imbalance or pre-existing rhythm disturbance is difficult; moreover, arrhythmia can develop in myocarditis [17]. In a study by Peretto et al. [18], 78.7% of patients with confirmed myocarditis had some forms of ventricular arrhythmia. Thus, the pathophysiology of rhythm disturbance is affected by the stage of myocardial damage, and the characteristics of arrhythmia in acute and healed myocarditis vary.
Possible mechanisms of rhythm disturbance in COVID-19 are direct damage to cardiomyocytes with impaired plasma membrane integrity and electrical conductivity, pericardial infection and massive edema, ischemia due to microvascular pathology caused by pericyte infection, arrhythmias caused by myocardial fibrosis, and action of pro-inflammatory cytokines [19, 20]. The latter mechanism is based on inflammatory cytokines, including interleukin-6 (IL-6), displacing the desmosome protein plakoglobin from the membrane of cardiomyocytes [21]. This can result in arrhythmias because insufficient cell adhesion is hypothesized to harm the membrane, resulting in cell death and eventual fibrosis. One of the primary mechanisms of arrhythmogenic cardiomyopathies is a decrease in the surface expression of desmosome proteins is [22]. Evidence shows that patients with COVID-19 have higher serum concentrations of IL-6 [23], and the level of IL-6 correlated with the severity of the patient’s condition. Thus, COVID-19 is most likely to blame for rhythm disturbances, particularly if a patient has a genetic susceptibility. Notably, the first three scenarios can occur in active myocarditis, whereas the last two can occur in chronic or healed myocarditis.
COVID-19 predisposes a cohort of professional athletes to myocarditis. In turn, myocarditis, contributes significantly to the pathogenesis of sudden cardiac death in athletes. Physical activity can trigger life-threatening cardiac arrhythmias and affect immune function: moderate-intensity exercise can boost the immune system, whereas high-intensity exercise sharply lowers the immune response [24]. The condition of the muscle layer of the heart causes sudden death in acute myocarditis: the affected myocardium is predisposes a patient to ventricular arrhythmias; in chronic myocarditis, myocardial fibrosis contributes to ventricular arrhythmias by the formation of re-entry sites in adjacent areas. As a result, current guidelines recommend limiting physical activity for 3–6 months after a diagnosis of myocarditis [25].
Studies have reported myocardial infarction after COVID-19. Several possible mechanisms for this complication have been proposed, including viral envelope glycoprotein binding to porphyrin and the hemoglobin beta chain, which produces hypoxia and, as a result, type 2 myocardial infarction caused by an imbalance between myocardial oxygen demand and supply. In addition, we hypothesized that the prothrombotic condition associated with COVID-19 plays a role in the pathogenesis of myocardial infarction, which may contribute to the development of type 1 myocardial infarction associated with atherosclerotic plaque instability [26]. However, evidence shows that patients with COVID-19 have higher levels of thrombophilia than hospitalized patients with severe pneumonia of another etiology, indicating the likely role of other mechanisms [27].
COVID-19-related cardiac lesions often result in heart failure. Possible causes include direct viral damage to the myocardium, inflammatory damage, imbalance between oxygen demand and supply, and increased atherothrombosis caused by plaque instability. Prior cardiac disease is a predictor of more severe clinical manifestations and increased mortality in these patients [28].
Takotsubo syndrome, characterized by high troponin and brain natriuretic hormone (NT-proBNP) levels, T wave inversion, and ST segment elevation on the electrocardiogram, as well as signs of ballooning of the middle and apical segments of the myocardium according to imaging, is a less common cardiac manifestation of COVID-19. Psycho-emotional stress is the most prevalent cause of takotsubo cardiomyopathy, which results in the release of catecholamines, which is not uncommon in a pandemic [17].
The main mechanisms of structural heart damage and the clinical manifestations they cause are summarized in Fig. 1.
Fig. 1. Main mechanisms of structural damage in the heart of patients with COVID-19 and the clinical manifestations they cause.
Diagnosis of myocarditis, including COVID-19-associated myocarditis
In clinical practice, diagnosing myocarditis can be difficult. In 2013, the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases established presumptive and definite criteria for myocarditis diagnosis. Myocarditis is suspected based on the clinical presentation (chest pain), electrocardiogram findings (ST segment elevation), laboratory findings (e.g., high troponin), and imaging findings such as echocardiography and magnetic resonance imaging (MRI) [29]. The effectiveness of the latter technique is also emphasized in Russian guidelines for myocarditis diagnosis [30].
MRI is a valuable tool for diagnosing myocarditis according to the Lake Louise Criteria, originally published in 2009, which at that time included the evaluation of signs such as signal hyperintensity on T2-weighted imaging (T2WI), short T1 inversion recovery, and delayed non-coronary contrast uptake [31]. The use of the original Lake Louise Criteria was limited because of the subjectivity of the qualitative assessment of the above signs. Therefore, in 2018, the criteria were revised and supplemented with parametric mapping, allowing us to measure the regional and global T1 and T2 relaxation times of the myocardium and the extracellular volume (ECV). The 2018 Lake Louise Criteria have higher sensitivity and specificity (88% and 96%, respectively) [32]. On MRI, inflammatory structural heart damage can be confirmed if at least one criterion is present in each of the two categories: T2WI signs of myocardial edema (myocardial hyperintensity or elevated T2) [33] and T1WI signs of myocardial damage (non-ischemic pattern of delayed contrast uptake or elevated T1 and/or ECV) [34]. If only one marker is present, inflammatory myocardial damage is still considered in the presence of relevant clinical and/or laboratory signs; however, the specificity of MRI is lower in this scenario. Systolic dysfunction (hypo- or akinesia areas) and pericarditis (contrasted pericardial layers) are additional (but not mandatory) signs. These criteria should only be used if there’s a clinical suspicion of inflammatory cardiac lesion, not as a screening strategy in asymptomatic cases. Furthermore, MRI helps in identifying important factors associated with myocarditis, such as signs of cardiomyopathies, which can all worsen a patient’s prognosis [35].
A crucial component of diagnosis is the need to rule out coronary artery lesions, particularly when the clinical picture does not rule out coronary heart disease [36]. However, even ruling out coronary artery disease does not allow us to be certain that the clinical symptoms are caused by myocarditis; in addition, various types of non-coronary myocardial damage exist. Endomyocardial biopsy is frequently required, which also helps in discovering the causative factor of the damage. Despite this, biopsy is rarely performed in patients who do not have heart failure and/or imminent cardiac arrhythmias because of its invasiveness and complications. According to current guidelines of the American Heart Association, a biopsy is required in patients with new-onset heart failure and hemodynamic instability to rule out giant cell or eosinophilic infiltration, for which there is a specific therapy; if untreated, the prognosis is usually poor [37].
Histological findings of biopsy and autopsy of the heart for myocarditis were summarized in the Dallas criteria (Dallas, 1985) and defined as histological evidence of inflammatory infiltrates in the myocardium associated with non-ischemic degeneration or necrosis of cardiomyocytes [38]. Myocarditis is classified as borderline when there is lymphocytic infiltration but no necrosis. However, in viral myocarditis, the Dallas criteria are not always effective: on average, the criteria for myocarditis are lacking in 50% of such cases with verified presence of a pathogen [39]. The Dallas criteria have recently been updated to include immunohistochemistry findings that allow for the presence of an aberrant inflammatory infiltration [36]. In addition, signs of non-ischemic degeneration and necrosis of myocytes must be observed.
The presence of an inflammatory infiltrate in the absence of cardiomyocyte necrosis is also possible in the normal myocardium [40]. Myocarditis is primarily determined by the nature of the inflammatory infiltrate (accumulation of inflammatory cells around myocytes with a predominance of lymphocytes over macrophages) and presence of myocardial necrosis. Referring to the most recent literature data, classifying myocarditis as infectious (the presence of a laboratory-confirmed pathogen) or non-infectious (the absence of such) is necessary. Because of the existence of non-infectious myocarditis, whether myocardial biopsy should be used to diagnose myocarditis has been controversial. Currently, this method is not used to diagnose myocarditis, particularly COVID-19-associated myocarditis; instead, diagnosis is based on circumstantial evidence, such as echocardiography or MRI abnormalities [41].
In general, the criteria for diagnosing myocarditis in COVID-19 remain the same; however, the path to this diagnosis may differ from that in other etiologies of myocarditis, which is mostly due to the need to protect healthcare professionals from infection.
Some laboratory parameters are suggestive of COVID-19-associated myocarditis, lymphocytopenia is common (up to 83% of patients), and as the disease progresses, the role of inflammatory markers (D-dimer, ferritin, and C-reactive protein) becomes more important [42]. High troponin levels are suggestive of possible myocardial damage and may indicate acute myocarditis. High troponin levels, combined with high levels of inflammatory markers, indicate a hyperinflammatory state and multiple organ failure [43]. Furthermore, high NT-proBNP levels may indicate hemodynamic overload [44].
Electrocardiographic markers of myocardial injury are not pathognomonic for myocarditis: numerous patterns, ranging from sinus tachycardia to ST elevation and T wave inversion, were reported [44].
Echocardiography is the first-line imaging modality in both stable and unstable cases with suspected myocarditis. However, this method has limitations in detecting myocarditis: for example, ventricular dysfunction can be caused by various other ischemic and non-ischemic disorders and is not a pathognomonic sign of myocarditis, and a normal left ventricular ejection fraction does not rule out myocarditis [45].
In general, cardiac lesion is determined by an increase in cardiac troponin levels, which occurs in 18%–28% of patients with COVID-19 [28]. As previously stated, detecting myocarditis in patients with COVID-19 is uncommon: for example, only approximately 7.8% of such cases were identified in a major review of 277 reports (22 studies) on the autopsy of the myocardium of patients who died from COVID-19. However, other histopathologies are associated with this disease: an inflammatory infiltration without signs of myocarditis was detected in 12.6% of cases, ischemia damage to individual cardiomyocytes in 13.7%, and acute myocardial infarction in 4.7% [46]. The reported 60% incidence of myocarditis on MRI in COVID-19 survivors contrasts strongly with the relatively low incidence of myocarditis at autopsy. For example, in a study of 15 patients with characteristic symptoms who recovered from COVID-19, signs of myocardial damage (edema, fibrosis, and ventricular dysfunction) were observed on MRI in 58% of cases [47]. Another study with a similar design showed that 78% of convalescents had MR signs of myocardial damage, whereas troponin levels were high in 75% of cases [48]. Even after recovery, the risk of cardiac lesion and subsequent ventricular dysfunction remains.
Scientists are actively working to develop fundamentally new methods for diagnosing myocarditis in cases where cardiac MRI does not always detect myocarditis due to method limitations, and myocardial biopsy is not recommended because of the inability to detect the virus in cardiomyocytes. These include detecting microRNA produced by T-helper type 17, which are active participants in acute myocardial damage and development of myocarditis and dilated cardiomyopathy in its aftermath. Researchers have already identified a new microRNA as a marker of myocarditis in murine models (with experimental autoimmune and viral myocarditis) and its human homolog. This marker allows differentiating myocarditis from myocardial infarction. Nonetheless, more questions require answers: for example, the greater variability in the level of this microRNA and whether this reflects the severity of the patient’s condition; and whether it will differentiate between conditions such as myocarditis and dilated cardiomyopathy [49]. Further research in this area will help in developing a method for non-invasive diagnosis of myocarditis.
During earlier coronavirus epidemics, cases of myocarditis were either not found (as with SARS) or were isolated (as with MERS) [50, 51]. The tropism of the COVID-19 pathogen to cardiomyocytes was generally because of the limited expression of ACE2 receptors on the surface of these cells. These and other findings suggest that endothelial cell activation, cytokine storm, electrolyte imbalance, and other potential immune mechanisms may directly cause myocardial damage in COVID-19 [46]. Furthermore, in the context of electrocardiographic abnormalities and positive troponin tests in normal coronary arteries, takotsubo syndrome should not be overlooked as a potential cause of myocardial damage [52]. In addition, myocarditis should be differentiated from other disorders such as sepsis-associated cardiomyopathy and acute coronary syndrome (particularly in fulminant myocarditis).
Myocarditis due to COVID-19 vaccination
Currently, no etiotropic therapy is available for COVID-19, and vaccination is the primary method of combating the pandemic; effective vaccines lower mortality rates dramatically. To date, the following vaccines are the most well-known worldwide: AstraZeneca/Oxford, Johnson and Johnson, Moderna, Pfizer/BioNTech, Sinopharm, Sinovac, and Sputnik V. Several vaccine principles have been proposed, including RNA- and DNA-based preparations (i.e., an approach that uses genetically modified RNA or DNA to create a protein that triggers an immune response, and vector-based preparations (i.e., using a harmless virus that cannot cause disease but serves as a platform for the production of coronavirus proteins that trigger an immune response), inactivated vaccines (a weakened virus to trigger an immune response), and protein-based vaccines (safe proteins or protein fragments that mimic the COVID-19 pathogen to trigger an immune response). AstraZeneca/Oxford, Johnson and Johnson, and Sputnik V vaccines are based on DNA delivered via a non-replicating recombinant adenovirus vector system. Moderna and Pfizer/BioNTech vaccines are based on mRNA technology and lipid nanoparticle delivery system [53–56]. Vector-based vaccines, such as mRNA-based vaccines, boost SARS-CoV-2 S protein production, which is the principal target for neutralizing antibodies produced by natural infection or vaccination.
During the vaccination program, data on the side effects of vaccines are published over time. In some cases, these reactions are thought to cause increased platelet aggregation, thrombosis, and inflammation. A possible mechanism is the synthesis of freely circulating spike proteins that can interact with ACE2 receptors by cells in the body that are targeted by vaccines [57].
Myocarditis and pericarditis have been reported [58, 59] following COVID-19 vaccination (usually within 6 h to 4 days), predominantly in patients who received mRNA-based vaccines (Pfizer and Moderna). In Israel, two large retrospective studies have been conducted in patients who received the Pfizer vaccine. One of these studies, with >5.1 million participants (21 days after the first dose and 30 days after the second dose), discovered 136 cases of myocarditis; 95% had mild symptoms, and one case was fatal [60]. Such symptoms were observed to be common in young men. Another study in Israel examined the medical records of more than 2.5 million Pfizer vaccinated patients and discovered that myocarditis occurs in 2.3 per 100,000 people. However, in young people aged 16–29 years, the incidence was 10 per 100,000 people [61]. In addition, those infected with the COVID-19 pathogen have an 18.28 times higher risk of myocarditis than those who are not infected, i.e., a significantly higher risk of myocarditis than after vaccination (on average 3.24 times higher than in unvaccinated patients) [62]. Although the symptoms of myocarditis were observed close to the vaccination time and researchers ruled out other probable causes (i.e., vaccination was identified as the cause of myocarditis), the pathophysiology of COVID-19 remains unknown. Existing hypotheses that this is caused by a nonspecific inflammatory reaction or antibody cross-reactivity due to molecular mimicry are consistent with the fact that the condition improves on anti-inflammatory therapy [58].
CONCLUSION
COVID-19 has a high mortality rate, including in the presence of concomitant cardiac lesion; however, its mechanisms are still unclear. Although COVID-19-associated myocarditis is thought to be the primary cause, inflammatory myocardial damage is rarely detected in histological examinations in patients with confirmed COVID-19. The virus is rarely discovered in the heart tissues and is found in immune cells rather than cardiomyocytes. It is primarily identified in nonspecific inflammatory infiltrate of the myocardium. Given these findings, endomyocardial biopsy should not be routinely employed to detect myocarditis in COVID-19.
Inflammatory myocardial damage is also possible occurs following vaccination, particularly with mRNA-based vaccines. This complication is more common in young patients, with moderate symptoms in the majority of cases. A high incidence of myocarditis following vaccination is not a sensible reason to avoid it, particularly among young people.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. D.A. Filatova ― collection and processing of materials, writing the text of the manuscript; E.A. Mershina ― concept and design of the work; V.E. Sinitsyn ― concept and design of the work, approval of the final text of the manuscript.
Авторлар туралы
Daria Filatova
Lomonosov Moscow State University
Email: dariafilatova.msu@mail.ru
ORCID iD: 0000-0002-0894-1994
SPIN-код: 2665-5973
Ресей, Moscow
Elena Mershina
Lomonosov Moscow State University; Medical Research and Education Center of Lomonosov Moscow State University
Email: elena_mershina@mail.ru
ORCID iD: 0000-0002-1266-4926
SPIN-код: 6897-9641
MD, Cand. Sci. (Med), Associate Professor
Ресей, Moscow; MoscowValentin Sinitsyn
Lomonosov Moscow State University; Medical Research and Education Center of Lomonosov Moscow State University
Хат алмасуға жауапты Автор.
Email: vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-код: 8449-6590
MD, Dr. Sci. (Med), Professor
Ресей, Moscow; MoscowӘдебиет тізімі
- Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950
- Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007
- Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1–5. doi: 10.1001/jamacardio.2020.3551
- Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286
- Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447. doi: 10.1002/ehf2.12805
- Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828
- Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(12):1030. doi: 10.7326/L20-1206
- Buja LM, Wolf DA, Zhao B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;(48):107233. doi: 10.1016/j.carpath.2020.107233
- Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir Med. 2020;10(7):681–686. doi: 10.1016/S2213-2600(20)30243-5
- Alijotas-Reig J, Esteve-Valverde E, Belizna C, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev. 2020;19(7):102569. doi: 10.1016/j.autrev.2020.102569
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond Engl. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031
- Choudry FA, Hamshere SM, Rathod KS, et al. High thrombus burden in patients with Covid-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022
- Bangalore S, Hamshere SM, Rathod KS, et al. ST-Segment elevation in patients with Covid-19: A case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020
- Guglin ME, Etuk A, Shah C, et al. Fulminant myocarditis and cardiogenic shock following COVID-19 infection versus COVID-19 vaccination: A systematic literature review. J Clin Med. 2023;12(5):1849. doi: 10.3390/jcm12051849
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585
- Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001
- Peretto G, Sala S, Rizzo S, et al. Ventricular arrhythmias in myocarditis: Characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75(9):1046–1057. doi: 10.1016/j.jacc.2020.01.036
- Peretto G, Sala S, Rizzo S, et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019;16(5):793–801. doi: 10.1016/j.hrthm.2018.11.024
- Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078
- Asimaki A, Tandri H, Duffy ER, et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4(5):743–752. doi: 10.1161/CIRCEP.111.964890
- Gemayel C, Pelliccia A, Thompson PD. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2001;38(7):1773–1781. doi: 10.1016/s0735-1097(01)01654-0
- Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141
- Modica G, Bianco M, Sollazzo F, et al. Myocarditis in athletes recovering from COVID-19: A systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(7):4279. doi: 10.3390/ijerph19074279
- Eichhorn C, Biere L, Schnell F, et al. Myocarditis in athletes is a challenge: Diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13(2):494–507. doi: 10.1016/j.jcmg.2019.01.039
- Azevedo RB, Botelho BG, de Hollanda G, et al. Covid-19 and the cardiovascular system: A comprehensive review. J Hum Hypertens. 2021;35(1):4–11. doi: 10.1038/s41371-020-0387-4
- Klok FA, Kruip MJ, van der Meer HJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;(191):145–147. doi: 10.1016/j.thromres.2020.04.013
- Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017
- Larina OM. Cardiac magnetic resonance imaging in the diagnosis of acute myocarditis: A clinical case and review of the literature. Bulletin Radiol Radiol. 2014;(5):54–59. (In Russ).
- Arutyunov GB, Paleev FN, Moiseeva OM. Myocarditis in adults. Clinical recommendations 2020. Russ Cardiol J. 2021;26(11):4790. (In Russ). doi: 10.15829/1560-4071-2021-4790
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007
- Tijmes SF, Thavendiranathan P, Udell JA, et al. Cardiac MRI assessment of nonischemic myocardial inflammation: State of the art review and update on myocarditis associated with COVID-19 Vaccination Radiol Cardiothorac Imaging. 2021;3(6):e210252. doi: 10.1148/ryct.210252
- Srichai MB, Lim RP, Lath N, et al. Diagnostic performance of dark-blood T2-weighted CMR for evaluation of acute myocardial injury. Invest Radiol. 2013;48(1):24–31. doi: 10.1097/RLI.0b013e3182718672
- Galán-Arriola C, Lim RP, Lath N, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73(7):779–791. doi: 10.1016/j.jacc.2018.11.046
- Blagova OV, Pavlenko EV, Varionchik NV, et al. Myocarditis as a natural phenomenon in patients with primary non-compact myocardium: Diagnosis, treatment and impact on outcomes // Russ J Cardiol. 2018;(2):44–52. (In Russ). doi: 10.15829/1560-4071-2018-2-44-52
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33)2636–2648, 2648a–2648d. doi: 10.1093/eurheartj/eht210
- Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116(19):2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093
- Aretz HT. Myocarditis: The Dallas criteria. Hum Pathol. 1987;18(6):619–624. doi: 10.1016/s0046-8177(87)80363-5
- Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296
- Zhang M, Tavora F, Zhang Y, et al. The role of focal myocardial inflammation in sudden unexpected cardiac and noncardiac deaths: A clinicopathological study. Int J Legal Med. 2013;127(1):131–138. doi: 10.1007/s00414-011-0634-x
- Titov VA, Ignatyeva VS, Mitrofanova LB. Comparative study of informativeness of noninvasive methods for diagnosis of myocardial inflammatory diseases. Russ J Cardiol. 2018;23(2):53–59. (In Russ).
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet Lond Engl. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3
- Mehta P, McAuley DF, Brown M, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0
- Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail Rev. 2022;27(1):251–261. doi: 10.1007/s10741-021-10087-9
- Mele D, Flamigni F, Rapezzi C, et al. Myocarditis in COVID-19 patients: Current problems. Intern Emerg Med. 2021;16(5):1123–1129. doi: 10.1007/s11739-021-02635-w
- Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;(50):107300. doi: 10.1016/j.carpath.2020.107300
- Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557
- Blanco-Domínguez R, Sánchez-Díaz R, de la Fuente H, et al. A novel circulating MicroRNA for the detection of acute myocarditis. N Engl J Med. 2021;384(21):2014–2027. doi: 10.1056/NEJMoa2003608
- Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X
- Kawakami R, Sakamoto A, Kawai K, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577
- Voysey M, Clemens SA, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1
- Shiravi AA, Ardekani A, Sheikhbahaei E, et al. Cardiovascular complications of SARS-CoV-2 vaccines: An overview. Cardiol Ther. 2021;11(1):13–21. doi: 10.1007/s40119-021-00248-0
- Watad A, De Marco G, Mahajna H, et al. Immune-Mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 Vaccination: 5. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435
- Albert E, Aurigemma G, Saucedo J, et al. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16(8):2142–2145. doi: 10.1016/j.radcr.2021.05.033
- Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730
- Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737
- Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475
Қосымша файлдар