Comparison of multiparametric and biparametric magnetic resonance imaging protocols for prostate cancer diagnosis by radiologists with different experience
- Autores: Vasilev Y.A.1, Omelyanskaya O.V.1, Vladzymyrskyy A.V.1, Gelezhe P.B.1,2, Reshetnikov R.V.1, Gonchar A.P.1, Blokhin I.A.1, Abdullin I.I.1, Kieva I.N.3
-
Afiliações:
- Moscow Center for Diagnostics and Telemedicine
- Joint stock company “European Medical Center”
- Speransky Children’s Hospital
- Edição: Volume 4, Nº 4 (2023)
- Páginas: 455-466
- Seção: Original Study Articles
- ##submission.dateSubmitted##: 15.04.2023
- ##submission.dateAccepted##: 15.06.2023
- ##submission.datePublished##: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/322816
- DOI: https://doi.org/10.17816/DD322816
- ID: 322816
Citar
Resumo
BACKGROUND: Magnetic resonance imaging can detect clinically significant prostate cancer and diagnose extracapsular extension and cancer stage. A scanning protocol that includes only T2-weighted and diffusion-weighted images represents a viable alternative to multiparametric magnetic resonance imaging provided that the high diagnostic accuracy of the test is maintained. In recent studies, biparametric and multiparametric magnetic resonance imaging demonstrated slight differences in the diagnostic accuracy in detecting prostate cancer.
AIM: To compare the diagnostic accuracy of biparametric and multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer using PI-RADS v2.1 with magnetic resonance imaging-guided multifocal biopsy as the gold standard.
MATERIALS AND METHODS: This retrospective study initially processed the medical records of 126 patients. The inclusion criteria were as follows: presence of PI-RADS 2.1 multiparametric magnetic resonance imaging, clinical information on free and bound prostate-specific antigen blood levels, a multifocal prostate biopsy performed, and a time interval between magnetic resonance imaging and biopsy of no more than 14 days. Three investigators (radiologists with <2, 2–5, and >5 years of experience) independently evaluated biparametric magnetic resonance imaging of the prostate for the presence of pathological foci. After 2 weeks, the researchers evaluated the multiparametric magnetic resonance imaging dataset of the prostate. Each lesion detected, starting from PI-RADS category 3, was compared with the result of a multifocal fusion biopsy. The biopsy result was presented as a sum of Gleason scores, and a Gleason score of ≥7 was considered clinically relevant. According to magnetic resonance imaging data, findings meeting PI-RADS criteria 4 and 5 were considered tumor foci.
RESULTS: The best values of sensitivity and specificity of foci detection on magnetic resonance imaging of the prostate gland were 62.5% and 74.6%, respectively. The highest diagnostic accuracy achieved was 70.1%. Magnetic resonance imaging had higher specificity rates for detecting prostatic foci when interpreted by radiologists with 2 years and >5 years of experience.
CONCLUSION: Both biparametric and multiparametric magnetic resonance imaging of the prostate demonstrated suboptimal diagnostic accuracy. The sensitivity and specificity of the method tended to improve with increasing experience of the radiologist. Biparametric protocols of prostate scanning have a definite economic advantage over multiparametric protocols because of the absence of contrast agents and consumables and a significant decrease in magnetic resonance scanner loading time; however, their use can lead to a decrease in the diagnostic accuracy of the method.
Palavras-chave
Texto integral
List of Abbreviations
DWI: diffusion-weighted imaging
DCE: dynamic contrast enhancement
MRI: magnetic resonance imaging
bpMRI: biparametric magnetic resonance imaging
mpMRI: multiparametric magnetic resonance imaging
T2WI: T2-weighted imaging
SS-EPI: single-shot echo planar imaging
TSE: turbo-spin echo
BACKGROUND
Multiparametric magnetic resonance imaging (mpMRI), which includes T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast enhancement (DCE) sequences, is critical in the clinical assessment of patients with high prostate-specific antigen (PSA) levels.
MRI can be used to diagnose clinically significant prostate cancer, detect extracapsular extension, and determine the disease stage. In 2019, the American College of Radiology and the European Association of Urology (European Symposium on Urogenital Radiology, ESUR) released the Prostate Imaging Reporting and Data System, version 2.1 (PI-RADS v2.1) for standardizing MRI data acquisition and image interpretation [1].
Since 2020, the American Urological Association and European Association of Urology have recommended the use of mpMRI for biopsy-naïve men who were suspected of prostate cancer [2, 3]. A clinical study by O. Rouvière et al. [3] showed that 27% of men with high PSA levels could avoid an unnecessary biopsy using mpMRI. Since most men undergo PSA testing at least once in their lifetime, these guidelines have resulted in a marked increase in demand for prostate MRI.
Assigning a PI-RADS assessment category relegates the use of DCE imaging to a minor role because it is only used for the differential diagnosis between PI-RADS 3 and 4 lesions in the peripheral zone. In addition, the use of DCE poses a risk of nephrogenic systemic fibrosis in patients with renal insufficiency. Therefore, interest in parametric MRI (bpMRI) is growing, which is an abbreviated prostate MRI protocol that excludes DCE imaging [4–6].
Owing to its high diagnostic accuracy, the bpMRI protocol, a combination of T2WI and DWI sequences, is emerging as a viable alternative to mpMRI [7]. Recent studies have shown minor differences in the diagnostic accuracy between bpMRI and mpMRI for detecting prostate cancer [6]. Efforts to create a bpMRI protocol have been successful in demonstrating intensity nonuniformity, resolution, and nonlinearity comparable to those of mpMRI [8].
The growing interest in bpMRI has encouraged the PI-RADS Steering Committee to issue a consensus statement calling for a higher-quality data before making evidence-based recommendations on bpMRI as an initial diagnostic work-up [9].
The aim of this study was to compare the diagnostic accuracy of bpMRI with that of mpMRI in detecting clinically significant prostate cancer based on PI-RADS v2.1 using targeted MRI/transrectal ultrasound (TRUS) fusion-guided prostate biopsy (number of points) as the gold standard.
The objectives of this study were to identify the sensitivity and specificity of mpMRI in comparison with bpMRI in diagnosing clinically significant prostate cancer (PI-RADS ≥4). In addition, the study compared the sensitivity and specificity of mpMRI with those of bpMRI images assessed by radiologists with different levels of experience. Finally, the interobserver agreement between radiologists with different levels of experience in assessing mpMRI and bpMRI images was evaluated.
MATERIALS AND METHODS
Study Design
This was an observational, single-center, retrospective extrapolation study.
Eligibility Criteria
Inclusion criteria: availability of a PI-RADS 2.1 mpMRI scan, clinical laboratory values of blood-free and bound PSA levels, and targeted MRI/TRUS fusion biopsy. Biopsy must be performed within 14 days after MRI.
Noninclusion criteria: image artifacts on the prostate MRI scan or MR images not compliant with PI-RADS 2.1, absence of one or more clinical markers, and a time interval between mpMRI and biopsy of >14 days.
Exclusion criteria: significant mpMRI artifacts, which precluded an adequate assessment, and uninformative biopsies.
Following the above criteria, radiologists with <2 years or >5 years of experience excluded 19 patients from the sample, whereas those with 2–5 years of experience excluded 23 patients.
Study Site
Patients who underwent prostate MRI and TRUS fusion biopsy were recruited from the European Medical Center (a private medical institution).
Study Duration
The study analyzed electronic medical records from January 1, 2022, to June 1, 2022.
Medical Intervention
The medical records of 126 patients were analyzed. Prostate mpMRI was performed using a Siemens Magnetom Aera 1.5Т 4G (Germany) with a body coil. The scanning protocol included the following set of pulse sequences (Table 1). After unloading and anonymization, several DCE images were removed from the mpMRI sequences, resulting in a dataset of bpMRI sequences. Three investigators (radiologists with <2 years of experience, 2 –5 years of experience, and >5 years of experience) independently evaluated prostate bpMRI sequences for pathological lesions. The lesion was assigned a score from 1 to 5 (as instructed in PI-RADS v2.1, DWI was used for peripheral zone lesions, and T2WI for transition zone lesions); then, an overall prostate PI-RADS v2.1 score was determined. The reference method was prostate histopathology based on targeted MRI/TRUS fusion biopsy.
Table 1. Prostate multiparametric magnetic resonance imaging protocol
Pulse sequence | Slice orientation | TE/TR, ms | FOV, mm | Pixel size, mm | Slice thickness/overlap, mm | Estimated scanning time, min |
T2WI TSE | Sagittal | 120/3800 | 250 × 250 | 1 × 1 | 3/0.3 | 2:26 |
T2WI TSE | Axial | 110/3938 | 180 × 180 | 0.45 × 0.6 | 2.5/0 | 3:33 |
DWI SS-EPI | Axial | 87/2425 | 160 × 160 | 1.25 × 1.32 | 3/0.3 | 6:50 |
T2WI TSE | Coronal | 110/2500 | 160 × 160 | 0.38 × 0.42 | 2.5/0 | 4:50 |
DCE-T1WI, temporal resolution of 15 s | Axial | 2.3/4.6 | 250 × 250 | 0.9 × 1 | 3/0 | 5:46 |
CE-T1WI | Axial | 1.3/2.3 | 400 × 350 | 1.6 × 1.7 | 4/0 | 0:21 |
Notes. CE, contrast enhancement; DCE, dynamic contrast enhancement.
After 2 weeks, the investigators evaluated the prostate mpMRI dataset, which included a series of dynamic contrast enhancement images. MRI interpretation was conducted by investigators who were blinded to the biopsy results. According to PI-RADS 2.1 [1], early contrast enhancement allows for reliable differentiation between PI-RADS 3 and 4 lesions localized in the peripheral zone.
Primary Outcome
The prostate lesion identified by bpMRT or mpMRI should be consistent with the histopathological findings.
Outcome Reporting Method
The identified lesions were tabulated, specifying their zonal location based on the PI-RADS 2.1 sector map. The central zone and anterior fibromuscular stroma were excluded from the assessment.
Each identified lesion of PI-RADS ≥3 was compared with the findings of targeted MRI/TRUS fusion biopsy. MRI/TRUS fusion biopsy overlays a prostate ultrasound on the saved prostate MR images (typically, axial T2WI). The biopsy sites were targeted and tracked on the obtained three-dimensional reconstruction of the prostate.
The biopsy findings were presented as the total Gleason score [10]. A total Gleason score of ≥7 is considered clinically significant. PI-RADS 4 and 5 MR images were consistent with malignant lesions.
Ethics Review
This study was approved by the Local Ethics Committee of the European Medical Center (Minutes of the Meeting No. 1 of April 24, 2023).
Statistical Analysis
For each dataset, the experts separately calculated the diagnostic power parameters, including the Youden index. Interobserver agreement between radiologists was estimated as percentages and Fleiss kappa.
Calculations were performed using R software version 4.1.31 using irr2 and dpyr packages3.
RESULTS
Study Subjects (Participants)
Radiologists with <2 and >5 years of experience analyzed a total of 107 patient datasets, whereas radiologists with 2–5 years of experience analyzed 103 patient datasets.
Key Findings
The highest sensitivity and specificity of bpMRI for detecting pathological lesions in the prostate were 70.0% and 67.2%, respectively. The highest sensitivity and specificity of mpMRI for detecting pathological lesions in the prostate were 62.5% and 74.6%, respectively. No adverse events were reported.
The number of prostate lesions detected by radiologists with different levels of experience is presented in Table 2. The diagnostic accuracy of the radiologists is presented in Tables 3 and 4 for the bpMRI and mpMRI sequences, respectively. The interobserver agreement values are shown in Tables 5 (unit fractions) and 6 (Fleiss kappa).
Table 2. Absolute and relative number of prostate lesions detected by radiologists with different levels of experience, n (%)
Level of experience, years | Protocol | True positive | True negative | False-positive | False negative |
<2 | bpMRI | 19 (17.8) | 47 (43.9) | 20 (18.7) | 21 (19.6) |
mpMRI | 19 (17.8) | 52 (48.6) | 15 (14.0) | 21 (19.6) | |
2–5 | bpMRI | 31 (29.8) | 23 (22.1) | 42 (40.4) | 8 (7.7) |
mpMRI | 32 (30.8) | 19 (18.3) | 46 (44.2) | 7 (6.7) | |
≥5 | bpMRI | 28 (26.2) | 45 (42.1) | 22 (20.6) | 12 (11.2) |
mpMRI | 25 (23.4) | 50 (46.7) | 17 (15.9) | 15 (14.0) |
Notes. bpMRI/mpMRI, biparametric/multiparametric magnetic resonance imaging.
Table 3. Comparison of the PI-RADS 2.1 diagnostic criteria for prostate lesions using biparametric magnetic resonance imaging by radiologists with different levels of experience
Level of experience, years | Sensitivity | Specificity | Accuracy | Prognostic value | Youden index | |
Positive | Negative | |||||
<2 | 47.5 (31.5–63.9) | 70.2 (57.7–80.7) | 61.7 (51.8–70.9) | 48.7 (36.8–60.8) | 69.1 (61.6–75.8) | 0.177 |
2–5 | 79.5 (63.5–90.7) | 35.4 (23.9–48.2) | 51.9 (41.9–61.8) | 42.5 (36.7–48.4) | 74.2 (58.8–85.3) | 0.149 |
≥5 | 70.0 (53.5–83.4) | 67.2 (54.6–78.2) | 68.2 (58.5–76.9) | 56.0 (46.1–65.5) | 79.0 (69.4–86.1) | 0.372 |
Notes. The values are presented as the median (Me) and 95% confidence interval (95% CI).
Table 4. Comparison of the PI-RADS 2.1 diagnostic criteria for prostate lesions using multiparametric magnetic resonance imaging by radiologists with different levels of experience
Level of experience, years | Sensitivity | Specificity | Accuracy | Prognostic value | Youden index | |
Positive | Negative | |||||
<2 | 47.5 (31.5–63.9) | 77.6 (65.8–86.9) | 66.4 (56.6–75.2) | 37.4 (28.2–47.3) | 55.9 (42.2–68.8) | 0.251 |
2–5 | 82.1 (66.5–92.5) | 28.2 (18.6–41.8) | 49.0 (39.1–59.0) | 41.0 (35.9–46.3) | 73.1 (55.7–85.4) | 0.113 |
≥5 | 62.5 (45.8–77.3) | 74.6 (62.5–84.5) | 70.1 (60.5–78.6) | 59.5 (47.8–70.3) | 76.9 (68.6–83.6) | 0.371 |
Notes. The values are presented as the median (Me) and 95% confidence interval (95% CI).
Table 5. Interobserver agreement between radiologists (unit fractions)
Protocol Level of experience | bpMRI, <2 years | mpMRI, <2 years | bpMRI, >5 years | mpMRI, >5 years | bpMRI, 2–5 years | mpMRI, 2–5 years |
bpMRI, <2 years | 1 | 0.798 | 0.558 | 0.673 | 0.413 | 0.356 |
mpMRI, <2 years | 1 | 0.654 | 0.817 | 0.356 | 0.298 | |
bpMRI, >5 years | 1 | 0.808 | 0.442 | 0.452 | ||
mpMRI, >5 years | 1 | 0.413 | 0.357 | |||
bpMRI, 2–5 years | 1 | 0.904 | ||||
mpMRI, 2–5 years | 1 |
Notes. bpMRI/mpMRI: biparametric/multiparametric magnetic resonance imaging.
Table 6. Interobserver Agreement Between Radiologists (Fleiss’s kappa)
Protocol Level of experience | bpMRI, <2 years | mpMRI, <2 years | bpMRI, >5 years | mpMRI, >5 years | bpMRI, 2–5 years | mpMRI, 2–5 years |
bpMRI, <2 years | 1 | 0.669 | 0.318 | 0.482 | 0.195 | 0.136 |
mpMRI, <2 years | 1 | 0.446 | 0.693 | 0.129 | 0.087 | |
bpMRI, >5 years | 1 | 0.699 | 0.206 | 0.229 | ||
mpMRI, >5 years | 1 | 0.194 | 0.165 | |||
bpMRI, 2–5 years | 1 | 0.846 | ||||
mpMRI, 2–5 years | 1 |
Notes. bpMRI/mpMRI, biparametric/multiparametric magnetic resonance imaging.
DISCUSSION
Summary of the Key Findings
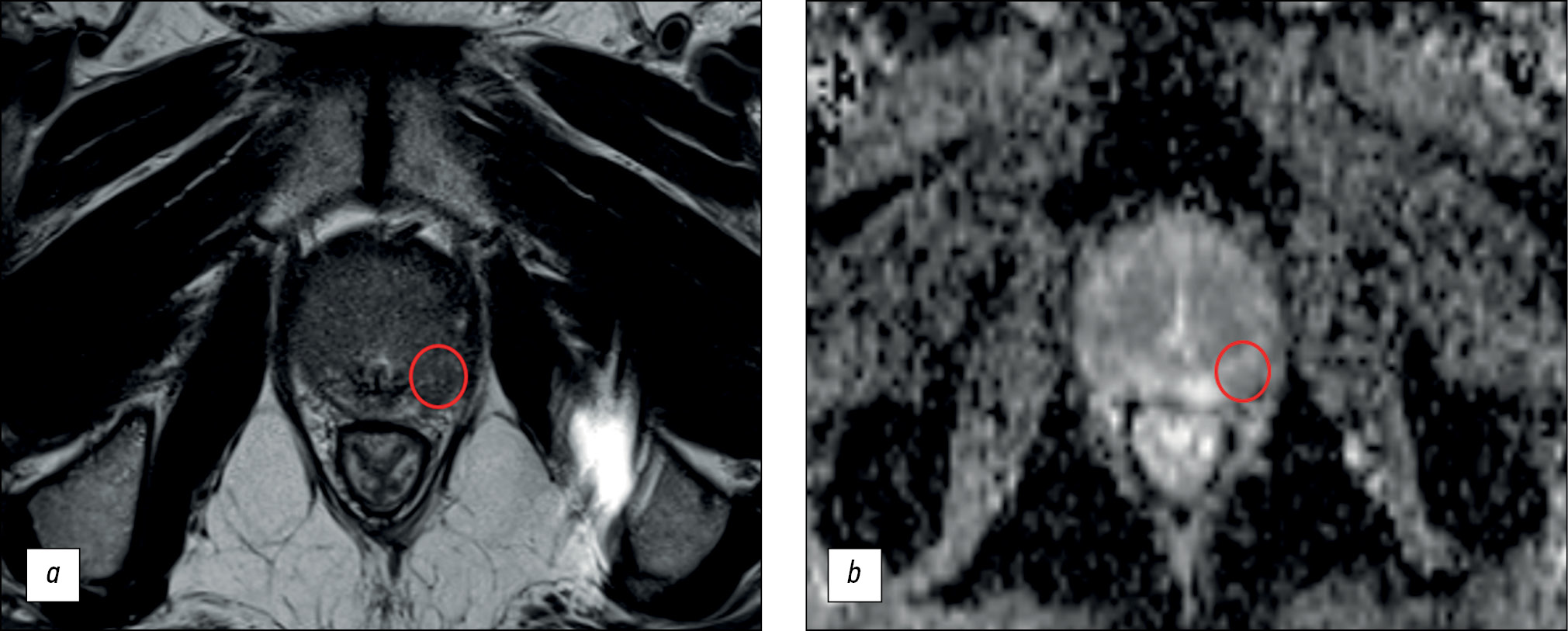
The main finding of our study is that the diagnostic power of prostate MRI is low. The maximum diagnostic accuracy for lesion detection was 70.1%, with a sensitivity of ≤62.5% and specificity of ≤74.6%. Based on the obtained values, MRI cannot be considered a reliable method for early diagnosis because of its suboptimal sensitivity (Fig. 1).
Fig. 1. An example of a false-positive result of biparametric magnetic resonance imaging: a ― T2-weighted image in the axial plane: in the lateral posterior segment of the peripheral zone of the left lobe in the middle part of the prostate gland there is a focus of reduced signal corresponding to the zone of diffusion restriction; b is a map of the measured diffusion coefficient. This lesion was characterized by the physician as PI-RADS5. According to multifocal biopsy, prostate tissue without signs of tumor growth.
This study also showed that mpMRI improved the diagnostic power of the method by increasing specificity. This is true when interpreted by radiologists with <2 (77.6% with mpMRI vs. 70.2% with bpMRI) and >5 years (74.6% with mpMRI vs. 67.2% with bpMRI) of experience.
Discussion of the Key Findings
The results obtained are consistent with those published in the scientific literature worldwide. J. Wallström et al. [6] reported that the mpMRI scan identified one additional case compared with the bpMRI (84 vs. 83 cases, respectively). In a retrospective study by C.K. Kuhl et al. [7], mpMRI detected an additional 10 out of a total of 329 cancers. In a prospective study by J.P. Zawaideh et al. [11], bpMRI identified 116 cases, whereas mpMRI identified 117 cases. In meta-analyses, Z. Kang [12] and X.K. Niu [13] reported similar diagnostic accuracy of bpMRI and mpMRI in detecting prostate cancer.
Our findings are inconsistent with those of the classical PROMIS study [14], which demonstrated high sensitivity (93%) but low specificity (41%) of MRI. However, this study considered PI-RADS 3 lesions to be positive MRI results. The histological criteria for clinically significant prostate cancer differed because Gleason 3 + 4 lesions were excluded. The suboptimal diagnostic accuracy of MRI may be due to the abnormal distribution of normal cases and pathologies in our sample.
The main difference in mpMRI is the inclusion of DCE in the scanning protocol. This study demonstrates that DCE enhances the specificity of detecting prostate lesions by radiologists with <2 years and those with >5 years of experience (Tables 2 and 3; Fig. 2). However, radiologists with 2–5 years of background paradoxically experienced a decrease in specificity when evaluating the mpMRI datasets.
Fig. 2. An example of an increase in the PI-RADS category using dynamic contrast enhancement: a ― T2-weighted image in the axial plane: in the lateral posterior segment of the peripheral zone of the right lobe in the middle part of the prostate gland there is a focus of reduced signal corresponding to the zone of diffusion restriction; b - map of measured diffusion coefficient: this lesion was characterized by the physician as PI-RADS 3 using biMRI, but with dynamic contrast enhancement (c) the lesion shows early contrast enhancement, which allows it to be regarded as PI-RADS 4.
DCE imaging in prostate mpMRI has traditionally been limited by longer image acquisition times. This includes the time-consuming procedure of contrast administration, which involves preparing for the injection by catheterizing the patient. Longer analysis times for DCE images and higher software requirements are also important factors. However, DCE helped increase the diagnostic accuracy (66.4% vs. 61.7% for a radiologist with <2 years of experience and 70.1% vs. 68.2% for a radiologist with >5 years of experience).
The use of bpMRI is also supported by concerns over the long-term safety of gadolinium-based contrast agents. Small amounts of gadolinium may be retained in the brain and other tissues. Although newer macrocyclic contrast agents have not been reported to cause adverse effects in clinical practice for patients with normal renal function, MRI contrast agents should be used only when they provide significant diagnostic value [15], as demonstrated in this study.
As previously mentioned, DCE as part of mpMRI is used to distinguish between PI-RADS 3 and 4 lesions located in the peripheral zone of the prostate. Based on the Epstein criteria, a clinically insignificant cancer is defined as a Gleason score of ≤6, being organ-limited (TNM stage of <T3), and having a volume of <0.5 cm3, which must be confirmed histopathologically [16]. The same definition was used in PI-RADS v2.1 [1]. Identifying clinically insignificant tumors is crucial for active follow-up.
This study differs from those by the authors mentioned above [6, 7] in that it reports a decrease in the number of false-positive prostate tumors with DCE. As a result, this method had a higher positive prognostic value. J.P. Zawaideh et al. [11] obtained similar results.
If a lesion of the PI-RADS ≥3 is detected, DCE will not alter the approach to scheduling a prostate biopsy. It is important to consider that transrectal biopsy is an invasive procedure that carries the risk of infection and requires hospitalization [17].
Limitations
This study has significant limitations. The retrospective design of the study required the selection of patients who underwent fusion biopsy. Therefore, the distribution of normal cases and pathologies in our sample did not correlate with that of the general population. Sequential viewing of both bpMRI and mpMRI datasets by radiologists, even after the 2-week washout period, did not eliminate bias. The limited number of participating radiologists in the study prevented us from making a definitive conclusion about the consistency of their evaluations.
The interobserver agreement among experts with <2 and >5 years of work experience was moderate. However, the results were more consistent with the use of mpMRI. The literature presents varying data on the influence of radiologists’ experience on the diagnostic quality of both protocols. For instance, E.D. Campli et al. [18] found no significant effect, whereas M. Gatti et al. [19] demonstrated that radiologists with little experience evaluated bpMRI with less accuracy.
CONCLUSION
Regardless of the protocol used, prostate MRI demonstrated suboptimal diagnostic power. Although parametric prostate scanning protocols may have economic benefits over multiparametric ones because of the absence of costs for contrast agents and consumables and a significant reduction in the loading time of the MR scanner, their use may lead to a decrease in the diagnostic accuracy of the method.
The observed trend of increased sensitivity and specificity of the method with a higher level of radiologist experience highlights the importance of training in the interpretation of prostate MRI based on PI-RADS.
A prospective study is necessary to confirm the role of bpMRI in the early diagnosis of prostate cancer.
ADDITIONAL INFORMATION
Funding source. This article was prepared by a group of authors as a part of the research and development effort titled “Scientific evidence for using radiomics-guided medical imaging to diagnose cancer”, No. 123031400009-1”, (USIS No. 123031500005-2) in accordance with the Order No. 1196 dated December 21, 2022 “On approval of state assignments funded by means of allocations from the budget of the city of Moscow to the state budgetary (autonomous) institutions subordinate to the Moscow Health Care Department, for 2023 and the planned period of 2024 and 2025” issued by the Moscow Health Care Department.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Yu.A. Vasilev, O.V. Omelyanskaya, A.V. Vladzymyrskyy ― research concept; P.B. Gelezhe, R.V. Reshetnikov ― data analysis, text writing; A.P. Gonchar, I.A. Blokhin, I.I. Abdullin, I.N. Kieva ― data analysis.
1 R Project for Statistical Computing. Available at: https://www.r-project.org/.
2 irr: Various coefficients of interrater reliability and agreement. Available at: https://cran.r-project.org/web/packages/irr/index.html.
3 dplyr: Grammar of data manipulation. Available at: https://github.com/tidyverse/dplyr.
Sobre autores
Yuriy Vasilev
Moscow Center for Diagnostics and Telemedicine
Email: npcmr@zdrav.mos.ru
ORCID ID: 0000-0002-0208-5218
Código SPIN: 4458-5608
MD, Cand. Sci. (Med)
Rússia, MoscowOlga Omelyanskaya
Moscow Center for Diagnostics and Telemedicine
Email: o.omelyanskaya@npcmr.ru
ORCID ID: 0000-0002-0245-4431
Código SPIN: 8948-6152
Rússia, Moscow
Anton Vladzymyrskyy
Moscow Center for Diagnostics and Telemedicine
Email: npcmr@zdrav.mos.ru
ORCID ID: 0000-0002-2990-7736
Código SPIN: 3602-7120
MD, Dr. Sci. (Med), Professor
Rússia, MoscowPavel Gelezhe
Moscow Center for Diagnostics and Telemedicine; Joint stock company “European Medical Center”
Autor responsável pela correspondência
Email: gelezhe.pavel@gmail.com
ORCID ID: 0000-0003-1072-2202
Código SPIN: 4841-3234
Rússia, Moscow; Moscow
Roman Reshetnikov
Moscow Center for Diagnostics and Telemedicine
Email: reshetnikov@fbb.msu.ru
ORCID ID: 0000-0002-9661-0254
Código SPIN: 8592-0558
Rússia, Moscow
Anna Gonchar
Moscow Center for Diagnostics and Telemedicine
Email: a.gonchar@npcmr.ru
ORCID ID: 0000-0001-5161-6540
Código SPIN: 3513-9531
Rússia, Moscow
Ivan Blokhin
Moscow Center for Diagnostics and Telemedicine
Email: i.blokhin@npcmr.ru
ORCID ID: 0000-0002-2681-9378
Código SPIN: 3306-1387
Rússia, Moscow
Iskander Abdullin
Moscow Center for Diagnostics and Telemedicine
Email: iabdullin@emcmos.ru
ORCID ID: 0000-0003-1138-0822
Código SPIN: 6560-5219
Rússia, Moscow
Irina Kieva
Speransky Children’s Hospital
Email: kieva.irina@gmail.com
ORCID ID: 0000-0002-4060-5966
Código SPIN: 2279-9141
Rússia, Moscow
Bibliografia
- Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 Update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–351. doi: 10.1016/j.eururo.2019.02.0331
- Bjurlin MA, Carroll PR, Eggener S, et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol. 2020;203(4):706–712. doi: 10.1097/JU.0000000000000617
- Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncology. 2019;20(1):100–109. doi: 10.1016/S1470-2045(18)30569-2
- Boesen L, Thomsen FB, Nørgaard N, et al. A predictive model based on biparametric magnetic resonance imaging and clinical parameters for improved risk assessment and selection of biopsy-naïve men for prostate biopsies. Prostate Cancer Prostatic Dis. 2019;22(4):609–616. doi: 10.1038/s41391-019-0149-y
- Tamada T, Kido A, Yamamoto A, et al. Comparison of biparametric and multiparametric MRI for clinically significant prostate cancer detection with PI-RADS Version 2.1. J Magnetic Resonance Imaging. 2021;53(1):283–291. doi: 10.1002/jmri.27283
- Wallström J, Geterud K, Kohestani K, et al. Bi- or multiparametric MRI in a sequential screening program for prostate cancer with PSA followed by MRI? Results from the Göteborg prostate cancer screening 2 trial. Eur Radiol. 2021;31(11):8692–8702. doi: 10.1007/s00330-021-07907-9
- Kuhl CK, Bruhn R, Krämer N, et al. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology. 2017;285(2):493–505. doi: 10.1148/radiol.2017170129
- Abuladze LR, Semenov DS, Panina OV, Vasil AA. Optimized protocol of biparametric magnetic resonance imaging for prostate. Digital Diagnostics. 2022;3(3):166–177. doi: 10.17816/DD108484
- Schoots IG, Barentsz JO, Bittencourt LK, et al. PI-RADS committee position on MRI without contrast medium in biopsy-naive men with suspected prostate cancer: Narrative review. Am J Roentgenol. 2021;216(1):3–19. doi: 10.2214/AJR.20.24268
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125–128.
- Zawaideh JP, Sala E, Shaida N, et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: Assessment of contrast benefit in clinical practice. Eur Radiol. 2020;30(7):4039–4049. doi: 10.1007/s00330-020-06782-0
- Kang Z, Min X, Weinreb J, et al. Abbreviated biparametric versus standard multiparametric MRI for diagnosis of prostate cancer: A systematic review and meta-analysis. Am J Roentgenol. 2019;212(2):357–365. doi: 10.2214/AJR.18.20103
- Niu XK, Chen XH, Chen ZF, et al. Diagnostic performance of biparametric MRI for detection of prostate cancer: A systematic review and meta-analysis. Am J Roentgenol. 2018;211(2):369–378. doi: 10.2214/AJR.17.18946
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389(10071):815–822. doi: 10.1016/S0140-6736(16)32401-1
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium retention: A research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289(2):517–534. doi: 10.1148/radiol.2018181151
- Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol. 2011;60(2):291–303. doi: 10.1016/j.eururo.2011.05.006
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64(6):876–892. doi: 10.1016/j.eururo.2013.05.049
- Campli ED, Pizzi DA, Seccia B, et al. Diagnostic accuracy of biparametric vs multiparametric MRI in clinically significant prostate cancer: Comparison between readers with different experience. Eur J Radiol. 2018;(101):17–23. doi: 10.1016/j.ejrad.2018.01.028
- Gatti M, Faletti R, Calleris G, et al. Prostate cancer detection with biparametric magnetic resonance imaging (bpMRI) by readers with different experience: Performance and comparison with multiparametric (mpMRI). Abdominal Radiol (New York). 2019;44(5):1883–1893. doi: 10.1007/s00261-019-01934-3
Arquivos suplementares