Preparation for radioiodine therapy in patients with differentiated thyroid cancer: a modern perspective (a review)
- Autores: Reinberg M.V.1, Slashchuk K.Y.1, Trukhin A.A.1, Avramova K.I.1, Sheremeta M.S.1
-
Afiliações:
- Endocrinology Research Centre
- Edição: Volume 4, Nº 4 (2023)
- Páginas: 543-568
- Seção: Reviews
- ##submission.dateSubmitted##: 08.07.2023
- ##submission.dateAccepted##: 05.09.2023
- ##submission.datePublished##: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/532728
- DOI: https://doi.org/10.17816/DD532728
- ID: 532728
Citar
Resumo
Thyroid cancer is the most prevalent tumor of the endocrine system, accounting for 1%–3% of all malignant neoplasms as of 2021. Differentiated forms, papillary and follicular, with a relatively favorable prognosis, are detected in 90% of cases. The combination of surgical treatment, subsequent suppressive hormonal therapy, and radioiodine therapy provides a favorable prognosis in patients with differentiated thyroid cancer. However, an insufficient response to radioiodine therapy may be possible, which may be associated with multiple factors, including the preparation step for radioiodine therapy. To date, the question of choosing the optimal method of patient preparation remains relevant. This paper presents a review of the scientific literature on the preparation of patients with differentiated thyroid cancer for radioiodine therapy. The principles of preparation are based on the recommendations of leading expert societies, and publications related to this topic are highlighted and summarized, including the adverse events associated with radioiodine therapy, quality of life, efficacy, and long-term results of treatment. The main purpose of this review was to provide a comprehensive insight into the methods of preparing a patient with differentiated thyroid cancer for radioiodine therapy, highlight existing problems and promising areas of research, and modernize treatment toward personalized therapy. Scientific articles and reviews from the National Library of Medicine, Cochrane Library, and Google Scholar databases, published up to the end of January 2023, were searched by the keywords listed below in their various combinations. Recommendations from the following scientific communities were used: Russian Clinical Guidelines for Differentiated Thyroid Cancer, American Thyroid Association, European Thyroid Association, National Comprehensive Cancer Network, European Association of Nuclear Medicine, British Thyroid Association, and European Society for Medical Oncology. Articles not available in full, not in English or Russian, or systematic reviews of a similar topic, were excluded. In total, 124 sources were selected and analyzed, general tendencies of modern approaches to preparation for radioiodine therapy and actual problems were highlighted, concepts of optimization of preparation for radioiodine therapy within the framework of personalized therapy were covered, and results and conclusions were presented.
Texto integral
INTRODUCTION
Thyroid cancer (TG) has five histological types:
- Papillary (80%–85%)
- Follicular (10%–15%)
- Medullary (5%)
- High grade (1%)
- Anaplastic (0.1%–0.2%)
The first two types are low-grade cancers with relatively good outcomes. The global incidence rate of malignant neoplasms is between 1% and 3% of all new diagnoses.
Among thyroid nodules, up to 5% of cases (according to some data, up to 20%) are cancerous [1], whereas the average annual growth rate in incidence is 3%. Since 2011, the incidence has increased by 36%, whereas the mortality rate has remained low [2]. This is mainly due to improvements in diagnostic methods, including the increased availability and quality of ultrasonography.
Despite a good response to surgical treatment and radioiodine therapy (RIT), 20% of patients may experience a relapse of the disease, and in this case, an unfavorable prognosis is observed in 8% of cases [1]. In Russia in 2021, 996 patients per 100,000 population died from thyroid cancer. From 2011 to 2021, a statistically significant increase was found in the “crude” incidence of malignant thyroid neoplasms in children aged <15 years (40%) [2].
Patients with highly differentiated thyroid carcinoma (HDTC), including those at high risk of recurrence, have a generally favorable overall survival profile, and approximately 90%–95% were responders to radioactive iodine therapy [3]. The prognosis is somewhat worse in patients with distant metastases, incomplete response after the first course of radiotherapy, and advanced disease. According to various sources, the 10- and 5-year overall survival rates of such patients are approximately 30% and 55%, respectively [3, 4]. The tumor-specific survival rate is approximately 30%–65% [5]. A. Hassan et al. [6] reported 5-year disease-free survival rates of 52% in intermediate-risk patients and 17% in high-risk patients. Currently, no consensus has been established on the reasons for the incomplete response to RIT and thyroid cancer progression, which may be due to various factors, including the methodology and principles of preparing the patient for RIT. Finding the reasons for an incomplete response to therapy and developing methods to improve the quality of life and treatment approaches remain urgent problems.
RIT refers to a radical method of treatment for HDTC and is part of combination therapy in patients with predominantly intermediate and high risks of disease relapse (according to the criteria of scientific communities [7-11]). The goal of radionuclide therapy is the ablation of residual thyroid tissue after thyroidectomy and the removal of tumor tissue and metastases that can accumulate iodine-131 (I-131).
The effectiveness of RIT depends on various factors, including the histological type of the tumor, size of the primary tumor and/or metastases, presence of locoregional and/or distant metastases, patient’s age at the time of diagnosis, hormonal status of the thyroid gland at the time of HDTC detection, and RIT strategy. One of the important criteria is compliance with the conditions of preparation for RIT to optimize the uptake of I-131 by thyrocytes of residual tissues or thyroid cancer cells. Adequate thyroid-stimulating hormone (TSH) and low-iodine levels in the body are believed to be necessary for the adequate uptake of radiopharmaceuticals by tumor cells. These conditions are achieved through thyroid hormone withdrawal, injection of recombinant human thyrotropin alpha (rhTSH), and adherence to a low-iodine diet before RIT. However, no consensus has been reached on the timing and intensity of compliance with these recommendations regarding their effects on the long-term outcomes of therapy. In world practice (Table 1), the accepted standard for preparing for RIT includes the following steps:
- Discontinuation of levothyroxine sodium (LT4) for 3–6 weeks or
- Replacing LT4 with liothyronine (LT3) for 2 weeks followed by a 2-week withdrawal
- Use of rhTSH in patients at low and intermediate risk of relapse/progression
- Low-iodine diet for 1–4 weeks (with achievement of iodine concentration in single and/or daily urine <50–100 μg/L).
Table 1. Comparative characteristics of requirements and methods for radioiodine therapy in various scientific communities
Recommendations | Preparation strategy | Low-iodine diet | rhTSH | TSH before RIT | Iodine concentration |
Russian clinical guidelines [7] | Discontinuation of LT4 for 4 weeks or rhTSH (2 injections) | 2 weeks | Unspecified | >30 mIU/L | Unspecified |
European Association of Nuclear Medicine [8] | Discontinuation of LT4 in 3–4 weeks or LT4/LT3/rhTSH (2 injections) | 1–2 weeks | In low- or intermediate-risk patients or off-label in patients with distant metastases | >30 mIU/L | Adequate: <100 µg/L Optimal: <50 µg/L |
American Thyroid Association [9] | Discontinuation of LT4 in 3–4 weeks or LT4/LT3/rhTSH (2 injections) | 1–2 weeks | In low- or intermediate-risk patients | >30 mIU/L | Adequate: <100 µg/L Optimal: <50 µg/L |
European Thyroid Association [10] | Discontinuation of LT4 in 3–4 weeks or LT4/LT3/rhTSH (2 injections), preferably rhTSH | A diet may be prescribed; however, its benefits have not been clearly proven. It is recommended to discontinue iodine-containing medications | Not recommended in patients with distant metastases | >>30 mIU/L | Adequate: <100 µg/L Optimal: <50 µg/L |
European Society for Medical Oncology [11] | Discontinuation of LT4 in 4–5 weeks or rhTSH (2 injections) | Unspecified | Unspecified | >30 mIU/L | Unspecified |
British Thyroid Association [12] | Discontinuation of LT4 in 4 weeks or LT4/LT3/rhTSH (2 injections) | 1–2 weeks | Not recommended in patients with distant metastases and massive tumor spread beyond the thyroid capsule | >30 mIU/L | Unspecified |
The National Comprehensive Cancer Network [13] | Discontinuation of LT4 in 4–6 weeks or rhTSH (2 injections) | 10–14 days | Unapproved in patients with distant metastases | >30 mIU/L | < 100 µg/day |
Taking into account current trends, the contribution of each point of preparation for RIT must separately consider the patient’s quality of life, development of side effects, and effectiveness of RIT.
THYROID HORMONE WITHDRAWAL
As the first method of preparation for RIT, the LT4 regimen was discontinued 6 weeks before the initiation of RIT; however, this regimen led to severe hypothyroidism and related side effects. Subsequently, various variations were used to improve the quality of life without compromising the effectiveness of RIT. A. Golger et al. and T. Davids et al. concluded the sufficient adequacy of a 3-week withdrawal of LT4 in most patients [14, 15]. Alternatively, the option of replacing LT4 with LT3 for 2 weeks and then withdrawing LT3 for the same period can be used. However, according to some studies, this method does not provide additional benefits regarding the quality of life of patients [16, 17] and can sometimes potentiate the side effects of LT3 [18]. The limited availability of triiodothyronine preparations on the Russian market and the above factors may make this preparation option less convenient for patients.
Despite the proposed methods, 4 weeks of LT4 withdrawal or 2 weeks of LT3 withdrawal are sufficient for the development of clinically significant hypothyroidism, accompanied by associated side effects that significantly reduce the quality of life of patients. In addition, patients on suppressive therapy may tolerate symptoms of hypothyroidism less. Signs of hypothyroidism affecting the quality of life were reported to progress 2 weeks after cessation of suppressive therapy in most patients [19]. When analyzing the questionnaires, a deterioration in the quality of life was also noted 2 weeks after cessation of LT4 intake [20].
The issue of reducing the withdrawal time of LT4 to 2–3 weeks is being actively discussed. It can be effective in achieving the target TSH level and long-term treatment results.
Liel et al. analyzed 13 patients and reported that a TSH concentration >30 mIU/L was achieved in all patients an average of 17 days after LT4 cessation, and an exponential increase in TSH was observed [21].
R. Luna et al. studied TSH levels in 34 patients on days 4, 14, 21, and 28 after LT4 cessation, with an average of 20, 46, 75, and 112 mIU/L, respectively, corresponding to a linear increase in TSH levels. Thus, after 2 weeks, 75% of the patients achieved a TSH level >30 mIU/L, and 100% of the patients achieved this TSH level after 3 weeks of withdrawal [19].
According to A. Piccardo et al., the response to LT in the group with LT4 withdrawal in 2 weeks (n = 85) and 4 weeks (n = 137) did not differ, which was 82% over a 3–4-year follow-up period. However, the TSH level before RIT did not influence the incomplete therapeutic response [22]. Other authors have come to similar conclusions [23, 24].
Alternatively, P.W. Rosario et al. proposed a regimen with a reduction in the LT4 dose to 0.8 mg/(kg × day) 6–8 weeks before RIT, which was associated with the leveling of hypothyroidism that occurs during withdrawal, and this also made it possible to avoid the use of expensive rhTSH. Therefore, in 24 patients on the classic protocol, 71% noted a health deterioration, whereas in 27 patients on the reduced protocol, only 23% had symptoms of hypothyroidism. Laboratory parameters were also better in the second group. An increase in creatinine was noted in 63% of cases with the classic protocol compared with 30% with the reduced regimen, whereas 60% of patients noticed a difference in the various preparation methods, and 100% would prefer the reduced protocol if TSH stimulation was again necessary. The effectiveness rates of RIT were 75% and 79%, in the reduced and classic protocols, respectively [25].
This protocol has not received much attention from clinicians in other countries because the study was limited by a small sample size and previous RIT. However, this method can be considered in the preparation of low- and intermediate-risk groups for diagnostic procedures and RIT, and this issue requires further research.
Optimizing the preparation of patients for RIT is a current area of research. The above papers suggest that the duration of LT4 withdrawal can be reduced to 2–3 weeks without a decrease in the effectiveness of RIT. This may lead to a decrease in the risk of clinically significant hypothyroidism and an improvement in the quality of life of patients because signs of hypothyroidism in most patients start to progress 2 weeks after the cessation of LT4 treatment.
IS TSH CONCENTRATION >30 MIU/L AN OUTDATED DOGMA?
Controversy exists regarding the optimal TSH concentration before RIT in residual thyroid tissue. The efficiency of I-131 uptake by the tumor and residual thyroid tissue was assumed to depend on the level of expression of the sodium-iodine symporter (SIS), which in turn depends on the TSH concentration [26, 27]. D.Yu. Semenova et al. [28] showed that the average expression of SIS on the membrane of thyroid gland cells does not exceed 4.5%, and the maximum reaches 10%, whereas in normal thyroid tissue, the expression level was 30%–50%. More than 60% of patients with relapsed HDTC had an SIS expression level of <1%. Low SIS expression has been theorized to be an independent prognostic factor for the risk of relapse and disease severity; however, further research on this topic is required.
In 1977, C.J. Edmonds et al. first concluded that adequate uptake of I-131 by the tumor was impossible with TSH <30 mIE/L, and since then, this cutoff point has been used as an indicator of adequate patient readiness for RIT, also serving as a standard in most subsequent studies. Notably, in this study, not all patients achieved adequate I-131 uptake at the “target” TSH values, the sample size was small, and patients with distant metastases of thyroid cancer were also included, which could have a greater effect on the radiopharmaceutical uptake than the TSH concentration. Finally, these studies were not subjected to statistical analysis; thus, the conclusions were not considered unambiguous [26].
In 2021, J. Xiao et al. reported that a group of patients with a TSH concentration of 30–70 mIU/L showed better treatment outcomes than a group of patients with a concentration of ≤30 mIU/L at the time of RIT. Moreover, the effectiveness of RIT in the group with a TSH concentration of >70 mIU/L did not differ from that in the group with a TSH concentration of 30–70 mIU/L [29]. However, patients at high risk of disease relapse were excluded from the statistical analysis, and they comprised the majority of patients in the group with TSH <30 mIU/L and could respond worse to therapy because of the stage of thyroid cancer. Therefore, it is impossible to statistically reliably state that RIT is less effective based on TSH levels. Interestingly, 76% of patients achieved a TSH level of ~70 mIU/L by the end of week 4 of LT4 withdrawal, whereas 46% had a TSH concentration of >100 mIU/L. The authors concluded that due to the lack of additional benefit from achieving TSH concentrations >70 mIU/l (probably due to the presence of a certain threshold for rhTSH expression in the tumor cell), the timing of thyroid hormone withdrawal can be reduced.
T. Zhao et al. also reported the need to achieve TSH concentration >30 mIU/L in patients at low and intermediate risk; however, the study has its limitations: retrospective analysis, variability in I-131 activity (1.1–5.5 GBq), small sample size of patients with TSH <30 mIU/L, and short follow-up period [30].
In contrast to the above studies, an alternative opinion states achieving TSH concentration >30 mIU/L is unnecessary.
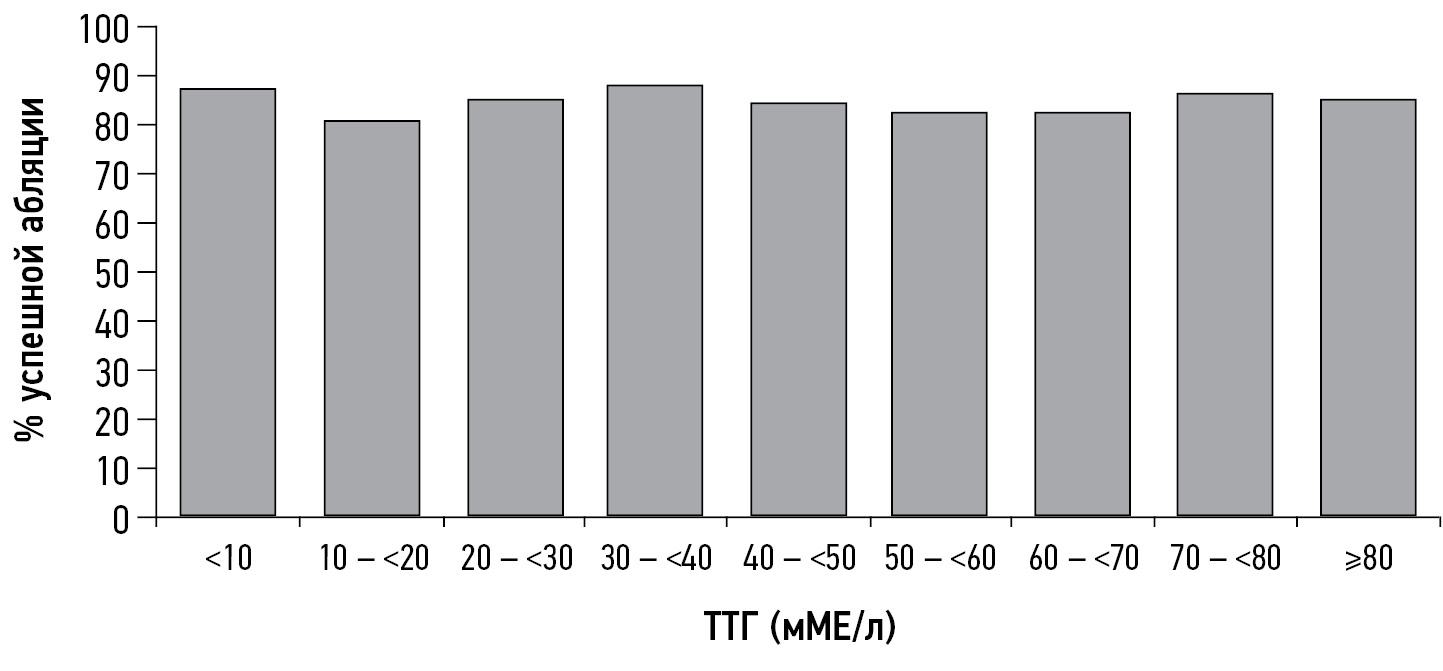
Z. Hasbek et al. observed 34 patients with an average median TSH concentration of 19.5 ± 6.0 mIU/L and 227 patients with TSH >30 mIU/L and noted that the lack of RIT effect was observed in one patient from the first group and 11 from the second group, which was not statistically significant. In non-responders, a significant increase in thyroglobulin levels and the presence of locoregional and distant metastases were observed. The authors concluded that TSH concentration is not the only and absolute factor for a successful response to RIT, whereas the patient’s age at diagnosis (>45 years), presence of metastases, thyroglobulin concentration, and residual thyroid tissue volume should be considered possible criteria for the low efficiency of RIT [31]. A research group from Germany came to similar conclusions; the TSH level at the time of ablation did not affect the percentage of successful ablation, disease-free survival, or tumor-specific mortality (Figure 1) [32].
Fig. 1. Percentage of patients with successful ablation relative to thyroid-stimulating hormone levels at the time of therapy I-131
In a retrospective analysis of 1873 patients without evidence of distant metastasis who underwent RIT, no statistically significant effect of TSH concentration was found on the effectiveness of RIT, disease-free survival, or HDTC-associated mortality. RIT was effective in 230 of 275 patients with TSH <30 mIU/L and 1359 of 1598 patients with TSH >30 mIU/L. At the time of ablation, incomplete response to RIT depended on the following factors:
- I-131 activity
- Histological characteristics
- Patient’s sex
- T-stage
- Presence of metastases in the regional lymph nodes
- Thyroglobulin concentration
The absence of metastases, low thyroglobulin concentration, smaller tumor size, high I-131 activity, and female sex were identified as independent factors for successful RIT. The authors also note that TSH levels are stimulated more slowly in patients:
In the absence of increased TSH concentrations to generally accepted target values (>30 mIU/L) in this group of patients after 3 weeks, further prolongation of thyroid hormone withdrawal was inappropriate. N. Ju et al. came to similar conclusions (Figure 2) [34].
Fig. 2. Percentage of patients with successful ablation of residual thyroid tissue with I-131 relative to thyroid-stimulating hormone levels. In 8 subgroups, no statistical significance was observed.
Slow TSH stimulation is probably associated with the influence of estrogens on the level of mRNA expression of the TSH beta-subunit, leading to its suppression in hyperestrogenism [35]. However, this mechanism of regulation of TSH concentration, as well as the theory about the influence of the estrogen status of the body on the incidence and progression of HDTC, are not fully understood and require further research [36-38].
Therefore, some factors can significantly influence the success of RIT in HDTC. They require attention and an individual approach, and with them, the dominant role of the “target” TSH concentration >30 mIU/L may be exaggerated. The study of RIT in a setting of TSH concentration <30 mIU/L will change views on modern aspects of preparation for RIT toward its greater safety with equivalent effectiveness.
RECOMMENDED HUMAN THYROTROPIN ALPHA
In 1987, rhTSH was obtained from the human TSH cell culture FRTL-5 of Chinese hamster ovaries. In 1998 and 2001, rhTSH was approved in the USA and Europe, respectively, as a preparation for diagnostic examinations with radioactive iodine. rhTSH was later approved as an alternative to thyroid hormone withdrawal when preparing patients for RIT:
- In Europe: since 2005
- In the USA: since 2007
- In Russia: since 2018
Numerous studies have shown rhTSH comparable to thyroid hormone withdrawal as a preparation for postoperative RIT [39–43]. However, the issue regarding the possibility of using rhTSH as part of RIT in patients at high risk of recurrent thyroid cancer and in the treatment of distant metastases remains open. Previously, several cases of ineffectiveness of RIT in high-risk patients when prepared with rhTSH have been documented, whereas repeated courses of RIT in a setting of thyroid hormone withdrawal were successful [44–46].
One of the proposed mechanisms is related to the different actions of the recombinant hormone and the endogenous hormone because of the greater sialylation of the molecule, different degrees of glycosylation of the TSH receptor, and polyclonality of tumors, which can develop with increasing number of RIT courses [46].
Currently, the question of whether the dose- and time-dependent effects of TSH (in other words, the area under the curve) on radiopharmaceutical uptake and treatment outcome is more significant than the “cutoff point” of 30 mIU/L. A. Vrachimis et al. suggested that this may be one of the limiting factors in the use of rhTSH [32].
Despite this fact, recent data indicate the same effectiveness of the use of rhTSH in patients not only at low and intermediate risk but also at high risk. In a retrospective study, J. Hugo et al. analyzed 586 patients (321 prepared by stopping LT4 treatment and 265 prepared using rhTSH), including intermediate risk and high-risk groups, and showed that long-term clinical outcomes with a median 9-year follow-up were not different. Moreover, in the short term (median, 2.5 years), the withdrawal group showed a statistically higher likelihood of incomplete response to primary RIT than the rhTSH group (47% vs. 39%, p = 0.03), with a higher rate of requiring repeat therapy, surgical intervention, or an RIT course (37% vs. 29%, p = 0.05). Economically, the use of rhTSH can shorten the period of active follow-up for patients with signs of persistent and/or recurrent disease [41] and reduce the economic budgetary costs [47–51], including a 70% probability of achieving economic benefits with a 30% reduction in the rhTSH cost [52].
Other researchers obtained similar results of at least equal effectiveness of rhTSH in the intermediate risk and high-risk groups [53-59].
In its HDTC guidelines, the American Thyroid Association does not recommend the use of the drug in patients at high risk of relapse [9]. Guidelines of the European Association of Nuclear Medicine allow the use of the drug off-label in patients with distant metastases [8].
The use of rhTSH is associated with fewer side effects. There remains some caution regarding the use of the drug in patients with metastases to the central nervous system because a strong TSH stimulation may lead to their growth/increase and severe clinical symptoms [60].
In a study of 88 patients prepared for RIT through thyroid hormone withdrawal and rhTSH (51 and 37, respectively), the 10-year survival rates were 62% and 73%, respectively. Therefore, the use of rhTSH was not associated with worse treatment outcomes or prognosis [61].
Table 2 summarizes the main advantages and disadvantages of using rhTSH and the target group of patients for its use.
Table 2. Advantages and disadvantages of using recombinant human thyrotropin alpha and its preferred indications
Advantages | Disadvantages | Target group |
Leveling the hypothyroidism phase is an opportunity to reduce the side effects on some risk organs Better quality of life compared with patients on withdrawal before and after RIT The preparation period for RIT/diagnostic studies is shorter Less risk of salivary gland damage Reducing the radiological load on the body as a whole (because of the absence of changes in GFR) and the risk of bone marrow damage Shorter possible hospitalization periods | Cost Higher incidence of damage to lacrimal gland ducts Lack of sufficient data on use in patients with distant metastases | Older age Chronic diseases of target organs that have a risk of exacerbation in the case of decompensated hypothyroidism (chronic heart failure, coronary heart disease of class II and higher, history of heart attacks/stroke, COPD, hepatitis, rheumatoid arthritis, diabetes mellitus, chronic kidney disease, mental illness, chronic pancreatitis, immunodeficiencies, etc.) Patients with a single/transplanted kidney Patients with carbohydrate metabolism disorders and obesity Patients with infections/diseases of the oral cavity, a history of sialadenitis, and stones in the ducts of the salivary glands Poorly controlled hypertension Non-alcoholic fatty liver disease and liver diseases in the stage of decompensation |
Despite the controversy regarding the use of rhTSH in the high-risk group, a potential benefit may be a greater increase in TSH levels in a shorter period. Patients with metastases have a lower expression of SIS, which may require a higher TSH concentration for I-131 uptake by tumor cells. In addition, long-term preparation by thyroid hormone withdrawal can negatively affect cancer prognosis and lead to progression [62-64].
I.I. Dedov et al. [65] showed that 70% of patients had a TSH concentration >100 mIU/L after the second rhTSH injection; however, no studies are currently conducted on the optimal TSH level in the high-risk group and its contribution to treatment effectiveness.
Separately, the advantages of rhTSH over LT4 discontinuation must be considered in terms of the effect on organs at risk, which will be discussed further.
SIDE EFFECTS WHEN USING DIFFERENT PREPARATION PROTOCOLS AND WAYS TO SOLVE THEM
When preparing for RIT, patients who are undergoing thyroid hormone withdrawal have severe iatrogenic hypothyroidism, accompanied by a decrease in the quality of life and side effects affecting target organs. Such effects are mediated by the presence of TSH receptors not only in thyroid tissues but also on the membranes of adipocytes, fibroblasts, osteoclasts, leukocytes, monocytes, amyocardiocytes, endothelial cells, and vascular smooth muscle cells, including the afferent glomerular arteriole [66].
In the cardiovascular system, the following are noted:
- Decreased ejection fraction
- Left ventricular diastolic dysfunction at rest
- Increase in total peripheral vascular resistance
- Endothelial dysfunction
All these factors may contribute to a decrease in the control of arterial hypertension in patients with essential hypertension [67]. Because of a decrease in the filtration function of the kidneys, the clearance of adrenaline, norepinephrine, and cortisol slows down [68]. Two studies have reported increased homocysteine levels [69, 70]. Such changes may contribute to the development and progression of the cardiorenal continuum. In patients who underwent thyroidectomy and were taking anticoagulants, an inverse correlation was found between TSH and INR levels during LT4 discontinuation, which may require additional monitoring of blood coagulation parameters to timely correct therapy.
Negative effects on the liver have been repeatedly reported; in patients with thyroid hormone withdrawal, increased activities of alanine aminotransferase and aspartate aminotransferase were reported [71, 72], whereas the use of rhTSH was not accompanied by impaired liver function [71]. Lipid metabolism is disturbed by an imbalance of high-density lipoproteins [67, 73]. This is because the lack of thyroid hormones leads to a decrease in the expression of receptors for high-density lipoproteins [74] and an increase in their concentration and an increase in total plasma cholesterol [73]. A definite connection has been established between thyroid dysfunction and affective disorders [75]. Moreover, disease control worsened as the intensity of hypothyroidism increased, which could be associated with reduced blood circulation in the brain and a diffuse [76] and/or regional [77] decrease in glucose clearance. The potentiation of the symptoms of depression, which most often accompanies hypothyroidism, is possible because of the insufficient ability of brain cells to receive adequate amounts of oxygen and glucose from the blood [78].
The causes of carbohydrate metabolism disorders may be increased evacuation capacity of the stomach and decreased transport of glucose by the liver, which leads to disturbances in both postprandial and fasting glycemia [79].
Evidence shows the influence of thyroid hormones on the modulation of the immune response [80], which in the case of hypothyroidism can lead to an increase in infectious morbidity. Particular attention has been paid to the suppression of renal functions, as proven in many studies [71, 81-87], including those that occur during LT4 discontinuation but not when using rhTSH. A study reported decreased renal perfusion on Doppler ultrasonography with rhTSH use. However, it was performed on a small sample of patients on day 5 after injection of the drug [66].
Cases of hyponatremia have been described following a low-iodine diet [88–91], with the following risk factors:
- Older age
- Treatment with thiazide diuretics
- Long duration of the low-iodine diet
- Long-term hypothyroidism
- Multiple metastases, which can contribute to the development of the syndrome of inappropriate antidiuretic hormone secretion, leading to its excessive increase [93, 94]
A common cause of hyponatremia was self-limitation of table salt by patients because of the low awareness of the principles of a low-iodine diet.
In a study by Horie et al., hyperkalemia developed in 5% of patients, correlating with age (over 60 years) and taking angiotensin-converting enzyme inhibitors, which could potentially also be associated with compromised renal function during long-term discontinuation of LT4 [93].
Interestingly, the choice of preparation method for RIT may also influence the frequency and intensity of side effects after exposure to I-131. Therefore, organs expressing SIS can accumulate I-131, which in some cases can lead to damage.
According to the clinical experience of our center, as well as worldwide publications, 20%–30% of the negative effects are related to the salivary glands [94-97]. Patients may experience taste changes, infections, facial nerve involvement, stomatitis, and candidiasis. The typical first symptom is obstructive swelling of the gland, resulting from the narrowing of the duct lumen associated with the inflammatory process. To prevent sialadenitis, many methods have been used, including the use of cholinomimetics, sialogogs, cytoprotectors (amifostine), and salivary gland massage. However, the efficacy was poor [97-100]. Moreover, the use of sialogogs on the first day after RIT leads to an increase in the radiation dose by approximately 28% in the salivary glands; therefore, the use of lemon/sucking sweets/other sialogogs on the first day after therapy is not recommended [99, 102]. If untreated, only 54% of patients were free of chronic sialadenitis after 6 years of follow-up [100], highlighting the need to find new methods to prevent sialadenitis.
In a study by A. Trukhin et al., rhTSH use was associated with a higher incidence of radiotracer accumulation in the lacrimal ducts than the 4-week discontinuation of LT4 [102]. According to other authors, the use of rhTSH reduced the number of cases of acute sialadenitis after RIT by approximately 20% [103], which may account for only 6.7% over the next year [104].
Secondary leukemia after ablation is one of the side effects of RIT that has not been widely recognized, but which deserves special attention. In a previous study, 148,215 patients were analyzed; the risk of developing acute and chronic myeloid leukemia in the first 3 years was higher and statistically significant in patients who underwent primary RIT for differentiated thyroid cancer compared with those who underwent surgical treatment only. Although the risk of acute myeloid leukemia rapidly declines to baseline levels by 3 years after RIT, the risk of chronic myeloid leukemia remains high for 10 years [105].
Another controversial finding is the increased number of stable chromosomal aberrations in patients after the administration of a low dose of I-131, which persisted longer in patients on LT4 discontinuation than in those on rhTSH [106]. The clinical interpretation of the results requires longer follow-up and a detailed search for cause-and-effect relationships.
Therefore, when preparing for RIT and monitoring patients, a clinician should probably be more cautious about patients with the following:
- Hypertension
- Immunodeficiency
- Moderate/severe dysfunction of the liver and/or kidneys
- Disturbances in electrolyte and/or carbohydrate metabolism
- Affective disorders
- Other previously described conditions
One of the methods to prevent and reduce the severity of side effects in susceptible organs associated with hypothyroidism is the preferential use of rhTSH in patients at risk of hypothyroidism complications and educating them on the basic principles of adhering to a low-iodine diet and regimen during RIT.
LOW-IODINE DIET
Based on the data collected to date, the degree of iodine uptake by tumor and normal thyroid cells is believed to be determined by the following:
- Volume of the residual thyroid tissue
- Adequate TSH stimulation
- SIS expression
- Median iodine concentration at the time of therapy [107]
Early studies have shown that iodine uptake by residual thyroid tissue increases 2–3-fold in patients following a low-iodine diet [108, 109], which may affect the effectiveness of RIT. Most scientific communities adhere to the following criteria when preparing a patient for RIT: optimal level of urine iodine excretion (UIE)<50 µg/L [8-10] and adequate level <100 µg/L [8]. However, no clear criteria have been established for the duration and intensity of adherence to a low-iodine diet.
To answer the question of whether a low-iodine diet is needed, several studies were conducted, including one by J. Tala et al., which caused a certain dissonance in the scientific community. The authors did not find a relationship between the urine iodine level and RIT effectiveness and suggested no differences between groups of patients with urine iodine levels of >100 µg/L and <100 µg/L. However, the study was conducted in a moderately deficient region (Siena, Italy), the sample of patients with high iodine content in the body was not sufficient, and the I-131 dose varied, which could greatly contribute to the clinical outcomes of RIT than moderate iodine deficiency [110].
The question of the optimal level of iodine in the body, after which the patient’s preparation for RIT is considered adequate, remains ambiguous.
M. Lee et al. did not find the difference in the effectiveness of RIT in the moderate and mild iodine deficiency groups [111]. A.E. Tobey et al. showed no significant difference in the RIT effectiveness between groups with iodine levels 50/100/150 mg/day; however, the risk of disease progression was higher in groups with urinary iodine levels >200 mg/day. To our knowledge, this is the first study to evaluate the relationship between pre-RIT iodine status and long-term clinical outcomes with a median follow-up of 3.7 years [112]. Other authors came to similar conclusions [45, 107, 113]. However, in a study by L.F. Morris et al., the success of RIT did not differ between groups with and without a low-iodine diet [114].
The diet duration is the next urgent issue. The most common recommended period is 1–2 weeks; however, approaches and protocols for a low-iodine diet vary from country to country. The timing and intensity of the diet could not be specified because of differences in the iodine supply of regions. A 2-week diet with restriction of iodine-containing foods can affect the quality of life, social functioning, and risk of hyponatremia. However, in regions with excess iodine intake, 2 weeks may be preferable to achieve adequate pre-ablation iodine levels in the body [107, 115-117]. An important point is to properly educate patients about the basic aspects of a low-iodine diet. In studies with minimal patient education by dietitians/nutritionists or dietary nurses, with the distribution of handouts, in some patients provided with a 3–7-day menu, better results were seen in the percentage reduction of iodine levels compared with baseline [112, 117-119].
In studies conducted in regions with moderate iodine deficiency or adequate iodine intake, optimal levels were achieved after a week of a low-iodine diet [118, 120] and after 4 days in studies by M.J. Pluijmen et al. and B.L. Dekker et al. [113, 121]. Some studies conducted in regions with high iodine intake have also shown the effectiveness of a weekly low-iodine diet [111, 112, 118, 119].
A limitation of many studies, other than the study by A.E. Tobey et al. [112], was that they have been conducted in the low- and intermediate-risk groups. This does not allow a full assessment of short- and long-term outcomes in the high-risk group. In studies conducted in countries with moderate iodine deficiency (e.g., Italy), with a median urinary iodine level of 95 µg/L in patients discontinuing LT4, values ranged from 25 to 1890 µg/L, which may affect the effectiveness of treatment in some cases. In this study, patients were not assigned to a low-iodine diet because of regional iodine deficiency status, and the high-risk group was excluded from the analysis.
Studying the patient’s iodine status before RIT is one of the methods for personalizing treatment. In each case, including those at high risk of disease relapse/progression, special attention should be paid to achieving an optimal iodine pool before RIT because each factor in the preparation process, including iodine status, can affect treatment success. The main studies on this topic are highlighted in Table 3.
Table 3. Comparative characteristics of a low-iodine diet and its effectiveness in countries with different iodine levels
1 | 2 | 3 | 4 | 5 | 6 | 7 |
Study | Patient sample (details) | Characteristics of the LID | Education | Method for assessing the iodine concentration/efficacy of RIT | Results (effects on RIT outcomes, % reduction in the iodine pool in the body) | Study limitations |
Brazil: moderate–high iodine intake | ||||||
R.P. Padovani et al. 2015 [122] | n = 125* | LID 15 days, n1 = 79; LID 30 days, n2 = 46 | + | 24-UIE | n1: M = 99 mg/L (60% reduction) n2: M = 80 mg/L (70% reduction) | *Most patients were excluded because of difficulty in complying with the protocol (initial, n = 306) |
Korea: excess iodine intake | ||||||
S.U. Sohn et al. 2013 [107] | n = 295 (single activity I-131 – 1100 MBq) | LID 2 weeks | +++ | UIE in a single urine sample adjusted for Cr | Successful ablation: 74.9% (221/295); in the group with UIE <66 µg/g (I/Cr ratio), the results were better than those in the group with UIE >66 µg/g: 81% versus 67%, p = 0.03; significantly lower results in the group with UIE >250 µg/g (p <0.05) | Retrospective analysis; patients with distant and cervical metastases were excluded, which could affect the statistics of RIT outcomes, without considering antithyroglobulin antibodies |
IDKS Yoo et al. 2012 [115] | n = 161: n1(SLID) = 90; n2(MLID) = 71 | SLID/MLID 2 weeks | ++ | UIE was not measured | Successful RIT: SLID, 75.8%; MLID, 80.3% (p = 0.48) | Patients with distant metastases were excluded. No information is available regarding patients receiving thyrotropin alpha |
H.K. Kim et al. 2011 [118] | n = 19 (on LT4 discontinuation) | SLID 2 weeks | +++ | UIE in a single urine sample, adjusted for Cr daily, 14 days | I/Cr ratio 0→7 day: ↓ from 576 to 26 µg/g 0→14 day: ↓ up to 19.6 µg/g By day 3: 95% I/Cr <150 µg/g By day 6: 95% I/Cr <66 µg/g | A single sample of urine was used to assess iodine excretion. The study results are not suitable for interpretation in iodine-deficient regions; the effectiveness of ablation has not been assessed |
C.Y. Lim et al. 2015 [117] | n = 101 n1(SLID) = 47; n2(MLID)=54 | SLID/MLID 4 weeks | ++ | 24-UIE, adjusted for Cr, weeks 2 and 4 | No statistical differences were found between n1 and n2. I/Cr ratio: Week 2: 28.6 µg/g Week 4: 35.0 µg/g. % I-131 uptake at weeks 2 and 4 did not differ between the groups | Short- and long-term outcomes of radioiodine therapy have not been assessed |
M. Lee et al. 2014 [111] | n = 195 | LID 2 weeks | +++ | 24-UIE at the end of weeks 1 and 2 | Week 1 - M =12.8 µg/L , 87.2% UIE <50 µg/L Week 2 - M=13.4 µg/L, 92.3% UIE <50 µg/L Successful ablation: 82.4%; no differences were found between the moderate and mild iodine deficiency groups | Various therapeutic activities (3700–7400 MBq) Exclusion of the high-risk group |
Italy: moderately deficient region | ||||||
J. Tala et al., 2010 [110] | n = 201 (n1 = 25, discontinuation of LT4 for 4 weeks; n2 = 76 on rhTSH) | Absent | – | UIE in a single urine sample | Successful ablation in 84.6% (UIE M=104 µg/L, from 25 to 1890 µg/L). No statistical differences were found between the groups with complete (M = 104 μg/L) and incomplete (M = 104 μg/L, 25 to 851 μg/L) responses. No difference was found in the response to therapy between the groups with UIE <100 µg/L and >100 µg/L (p = 0.98) | Retrospective analysis, small sample of patients with high iodine concentrations in urine, various levels of I-131 activity at the time of ablation (1100–5550 MBq), and no control group and high relapse group |
Netherlands: adequate iodine intake | ||||||
M.J. Pluijmen et al., 2003 [113] | n = 120: n(LID ) = 59 n (RD) = 61 | LID 4 days | +++ | 24-UIE: n (RD) = in 9 patients n (LID) = in 60 patients | n (LID): UIE avg. = 27 µg/day n (RD) UIE avg. = 159 µg/day I uptake in the thyroid region was higher in the LID group (5.1 ± 3.8 vs. 3.1 ± 2.5%, p < 0.001). Efficacy was higher in the LID than in the. RD group (71% and 45%, respectively) | Retrospective analysis Patients with metastases were excluded |
B.L. Dekker et al., 2022 [123] | n = 65 | – | +++ | 24-UIE on days 4 and 7 | Day 4: 24-UIE <50 µg in 72.1%, UIE avg. 36.1 g; Day 7: 24-UIE <50 µg in 82.0% (p = 0.18), UIE avg. 36.5 µg | These studies may not be applicable to countries with high iodine intake. Initial urinary iodine levels were not assessed |
USA: moderate-to-high iodine intake | ||||||
L.F. Morris et al., 2001 [116] | n = 94: n (LID ) = 44 n (RD) = 50 | LID 10–14 days RD: limit preparations with iodine, iodized salt, and seafood | ++ | UIE in a single urine sample (in seven patients on LID and in seven on RD) | Successful ablation in 68.2% (LID) versus 62% (RD), p = 0.53. In patients with metastases: 80.0% and 66.7. n (LID): ↓ I by 69.4% (UIE avg. 567.7 µg/L → 173.9 µg/L); n (RD): ↓ by 23.6% (UIE avg. 444.0 µg/L → 498.9 µg/L) | Various therapeutic activities (3700–7400 MBq) Small sample of patients for urine iodine screening The ablation criteria excluded the level of thyroglobulin or antibodies to thyroglobulin |
A.E. Tobey et al., 2018 [114] | n = 70 n1(rhTSH) = 16 n2 (discontinuation of LT4) = 54 | LID 2 weeks | +++ | 24-UIE | 21% had disease progression, and the risk was higher in patients with UIE >200 µg/day. Between groups with UIE 50, 100, 150 µg/day, no difference was found | Retrospective study and small sample I-131 activity from 1.1 to 11.1 GBq at RIT; Observation period of 3.7 years |
J.T. Park et al., 2004 [125] | n = 36 | n1 = 15: 2 weeks of LT4 + 2 weeks of LID; n2 = 21: 2 weeks LID without LT4 | + | UIE in a single urine sample at weeks 1 and 2, adjusted for Cr | I /Cr ratio: n1: M (week 1) = 76.91 µg/ g (21% UI <50; 71% <100 µg/g); n2: M (week 1) = 26.16 µg/g (78% <50 µg/g), p < 0.001 The UI after 2 weeks of LID did not differ between groups 1 and 2 (p < 0.15) | Short- and long-term outcomes of RIT were not assessed |
Japan: excess iodine intake | ||||||
S. Ito et al. 2018 [118] | n = 45 (single activity I-131 1100 MBq) | LID 2 weeks (SLID n = 12; LID n = 25) | +++ | UIE in a single urine sample adjusted for Cr | UIE (I/Cr): M before and after diet: 286 µg/ g (range, 40–7100 µg/g) and 74 µg/g (range, 16–816 µg/g), respectively. Successful ablation in 56% of the entire sample | Small sample; the effectiveness of RIT was assessed based on the results of scintigraphy without considering thyroglobulin and antibodies to thyroglobulin Patients with M1 were excluded. Complex interpretation for regions with moderate or adequate iodine intake |
C. Tomoda et al. 2005 [121] | n = 252: n 1(MLID) = 220 n 2(LID) = 15 n 3(SLID) = 17 | MLID = 1 week LID = 1 week SLID = 2 weeks | + | UIE in a single urine sample adjusted for Cr | n3(SLID): M I/Cr - 130 μg/ g (range 23–218 μg/g) n1(UYND): M I/Cr 125 μg/g (range 13–986 μg/g), (p <0. 01) I/Cr <100 µg/g - in 26% (n1) and 70% (n3) | Short- and long-term outcomes of radioiodine therapy have not been assessed. Iodine content was assessed in a single urine sample (not the “gold standard”) |
Malaysia: moderate iodine deficiency | ||||||
W.F. Sohaimi et al. 2019 [122] | N = 104 (LT4 discontinuation) | SLID/MLID = 1 week | + | UIE in a single urine sample | Day 0→7: UIE <100 µg/L in 89.1% (SLID) and 91.8% (MLID) MLID: UIE avg. 89.24 µg/L → 56.85 µg/L (↓ by 36.3%) SLID: UIE avg. — 107.8 µg/L → 63.82 µg/L (↓ by 40.8%) | Short- and long-term outcomes of radioiodine therapy have not been assessed |
Note:
“–”: lack of any education
“+”: printed instructions
“++”: printed and oral instructions
“+++”: printed, oral instructions, and training by healthcare personnel
24-UIE: daily urinary iodine excretion;
Cr: creatinine
I: iodine
LID: low-iodine diet
LT4: levothyroxine
M: median
MLID: moderate low-iodine diet
RD: regular diet
RIT: radioiodine therapy (first postoperative radioiodine therapy)
SLID: strict low-iodine diet
UI avg: average iodine levels in urine
UIE: urinary iodine excretion.
When considering adherence to a nearly stringent low-iodine diet protocol, no clear evidence was found to support a restrictive diet in either the degree of iodine reduction or the effectiveness of RIT. A more stringent protocol may be more associated with iodine reduction, lower quality of life, and psychological discomfort of patients. Therefore, the choice of a specific preparation protocol will depend on the ability of a particular site to inform/educate patients, presence of comorbid pathology, and initial iodine status of the region.
The 2020 Russian Clinical Guidelines recommend a 2-week low-iodine diet. Considering the iodine status of the region and world data, the diet duration can be reduced to 4–7 days.
CONCLUSION
To date, no consensus has been made regarding the indications for adjuvant RIT in patients with low and intermediate risk of recurrence, who represent the majority of patients with HDTC. The use of RIT carries a potential risk of complications; thus, the clinical benefit in each specific case must be assessed. This can only be done with the help of dynamic stratification of the risk of recurrence of thyroid cancer. The studies presented in the literature over the 80-year history of the method show the heterogeneity of options for the preparation and treatment of HDTC, which form the modern view of RIT.
In the low-/intermediate-risk group, performing RIT in an euthyroid state with a TSH level of <30 mIU/L may neutralize the risks associated with hypothyroidism and its complications. To date, limited but methodologically sound studies have demonstrated that achieving a pre-ablation TSH level >30 mIU/L is not necessary, as well as the efficacy of 2 weeks of LT4 discontinuation (compared with 4 weeks) to induce hypothyroid status. More studies are needed to extrapolate the results to a high-risk group. Theoretically, higher TSH levels may be required to stimulate SIS and improve the quality of therapy. The preferred drug for use in this patient population would be rhTSH; however, studies of the influence of its pharmacokinetic characteristics on the efficacy of RIT are required. In addition, its availability is currently limited by its high cost, which justifies the need to reduce the cost of its production technology in the Russian Federation and consider its availability to needy patient groups.
To increase the effectiveness of RIT, one of the steps in a personalized treatment approach may be to measure the iodine concentration in a single urine sample before RIT to assess the patient’s compliance with a low-iodine diet and predict the effectiveness of treatment. The procedure can be simplified by measuring the iodine concentration in saliva, as proposed by B.L. Dekker et al. [126]; although the information value is equivalent to the “gold standard” (iodine in 24-h urine sample), this method still needs to be validated and has certain limitations. An important contribution to the determination of patient management strategy can be made by determining the level of SIS expression as part of a standard pathological examination to predict the response to RIT, which may also influence the therapeutic activity of I-131.
The wide range of therapeutic activities of I-131 is one of the significant limiting factors in RIT preparation studies, affecting the final assessment of complex efficacy, which should be considered when carefully studying the issue addressed in the publication. The development of more precise and personalized approaches to RIT is based on an understanding of the many complex mechanisms, including individual patient characteristics, tumor biology, and other factors that underlie the efficacy and safety of this treatment. Careful selection of patients who will benefit from this intervention is necessary to minimize adverse events. The issue of the validity of prescribing RIT requires special attention and further study.
To summarize modern ideas and trends in the preparation for RIT, the following aspects are promising:
- Educate patients about the disease, RIT preparation, posttreatment regimen, and follow-up to improve quality of life.
- Reduce the period of a low-iodine diet to 4–7 days, eliminate significant dietary restrictions, or expand the dietary regime during the period of a low-iodine diet, depending on the iodine status of the region, and lower the “threshold” of optimal iodine level before ablation to 100–150 µg/L,
- Consider the possibility of performing RIT with a TSH level <30 mIU/L in some groups of patients (low/intermediate risk of relapse) by reducing the RIT withdrawal period from 4 to 2 weeks.
- Expand the indications for the use of rhTSH to include patients at high risk of relapse and those with significant comorbidities.
ADDITIONAL INFORMATION
Funding source. This work was conducted within the fund of the State Assignment “Study of pharmacosafety of theranostic radiopharmaceuticals using hybrid molecular imaging in the diagnosis and treatment of endocrine and oncological diseases in children and adults”. Registration number 123021000041-6.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. M.V. Reinberg — contribution to the concept of the paper, design of the review, collection and processing of materials, analysis of the obtained data and writing the text; K.Yu. Slashchuk — assistance in collection and processing of materials, data analysis, making substantial revisions to the manuscript to improve the scientific value of the article; approval of the final version of the manuscript; A.A. Trukhin — text editing, data analysis, adding valuable comments to improve the scientific value of the article; K.I. Avramova — assistance in collection and processing of materials, text editing; M.S. Sheremeta — adding valuable comments, approval of the final version of the manuscript.
Sobre autores
Maria Reinberg
Endocrinology Research Centre
Autor responsável pela correspondência
Email: mrezerford12@gmail.com
ORCID ID: 0009-0002-1632-2197
Researcher ID: IUO-4237-2023
Rússia, Moscow
Konstantin Slashchuk
Endocrinology Research Centre
Email: slashuk911@gmail.com
ORCID ID: 0000-0002-3220-2438
Código SPIN: 3079-8033
MD, Cand. Sci. (Med.)
Rússia, MoscowAlexey Trukhin
Endocrinology Research Centre
Email: Alexey.trukhin12@gmail.com
ORCID ID: 0000-0001-5592-4727
Código SPIN: 4398-9536
Cand. Sci. (Engin.)
Rússia, MoscowKarina Avramova
Endocrinology Research Centre
Email: dravramovak@gmail.com
ORCID ID: 0009-0008-4970-8911
Código SPIN: 4330-0263
Rússia, Moscow
Marina Sheremeta
Endocrinology Research Centre
Email: marina888@yandex.ru
ORCID ID: 0000-0003-3785-0335
Código SPIN: 7845-2194
MD, Cand. Sci. (Med.)
Rússia, MoscowBibliografia
- Vanushko VE, Tsurkan AYu. Treatment of differentiated thyroid cancer: cureunt statement of the problem. Clinical and experimental thyroidology. 2010;6(2):24–33. (In Russ). doi: 10.14341/ket20106224-33
- Kaprin AD, Starinskii VV, Shakhzadova AO, editors. Zlokachestvennye novoobrazovaniya v Rossii v 2021 godu (zabolevaemost’ i smertnost’). Moscow: MNIOI im. P.A. Gertsena −of NMRRC of the Ministry of Health of Russia; 2022. (In Russ).
- Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. The Journal of Clinical Endocrinology & Metabolism. 2006;91(8):2892–2899. doi: 10.1210/jc.2005-2838
- Cancer Stat Facts: Thyroid Cancer [Internet]. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. [cited 2023 Sep 1]. Available from: https://seer.cancer.gov/statfacts/html/thyro.html
- Braverman LE, Cooper DS, Kopp P. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 11th ed.. Philadelphia, PA: Wolters Kluwer; 2021.
- Hassan A, Razi M, Riaz S, et al. Survival Analysis of Papillary Thyroid Carcinoma in Relation to Stage and Recurrence Risk: A 20-Year Experience in Pakistan. Clinical Nuclear Medicine. 2016;41(8):606–613. doi: 10.1097/RLU.0000000000001237
- Well-differentiated thyroid cancer. Clinical guidelines. ID 329. Approved by the Scientific and Practical Council of the Ministry of Health of the Russian Federation. 2020. Available from: https://cr.minzdrav.gov.ru/recomend/329_1 (In Russ)
- Avram AM, Giovanella L, Greenspan B, et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. Journal of Nuclear Medicine. 2022;63(6):15N–35N.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020
- Pacini F, Fuhrer D, Elisei R, et al. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? European Thyroid Journal. 2022;11(1). doi: 10.1530/etj-21-0046
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30(12):1856–1883. doi: 10.1093/annonc/mdz400
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clinical Endocrinology. 2014;81 Suppl. 1:1–122. doi: 10.1111/cen.12515
- Haddad RI, Bischoff L, Ball D, et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2022;20(8):925–951. doi: 10.6004/jnccn.2022.0040
- Golger A, Fridman TR, Eski S, et al. Three-week thyroxine withdrawal thyroglobulin stimulation screening test to detect low-risk residual/recurrent well-differentiated thyroid carcinoma. Journal of Endocrinological Investigation. 2003;26(10):1023–1031. doi: 10.1007/bf03348202
- Davids T, Witterick IJ, Eski S, et al. Three-Week Thyroxine Withdrawal: A Thyroid-Specific Quality of Life Study. The Laryngoscope. 2006;116(2):250–253. doi: 10.1097/01.mlg.0000192172.61889.43
- Lee J, Yun MJ, Nam KH, et al. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010;20:173–179. doi: 10.1089/thy.2009.0187
- Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clinical Endocrinology. 2007;67(6):839–844. doi: 10.1111/j.1365-2265.2007.02972.x
- Rajamanickam S, Chaukar D, Siddiq S, Basu S, D’Cruz A. Quality of life comparison in thyroxine hormone withdrawal versus triiodothyronine supplementation prior to radioiodine ablation in differentiated thyroid carcinoma: a prospective cohort study in the Indian population. European Archives of Oto-Rhino-Laryngology. 2021;279(4). doi: 10.1007/s00405-021-06948-6
- Luna R, Penín M, Seoane I, et al. ¿Es necesario suspender durante 4 semanas el tratamiento con tiroxina antes de la realización de un rastreo-ablación? Endocrinología y Nutrición. 2012;59(4):227–231. (In Spanish). doi: 10.1016/j.endonu.2012.02.004
- Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997;7(4):613–619. doi: 10.1089/thy.1997.7.613
- Liel Y. Preparation for radioactive iodine administration in differentiated thyroid cancer patients. Clinical Endocrinology. 2002;57(4):523–527. doi: 10.1046/j.1365-2265.2002.01631.x
- Piccardo A, Puntoni M, Ferrarazzo G, et al. Could short thyroid hormone withdrawal be an effective strategy for radioiodine remnant ablation in differentiated thyroid cancer patients? European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(7):1218–1223. doi: 10.1007/s00259-018-3955-x
- Santos PA, Flamini ME, Mourato FA, et al. Is a four-week hormone suspension necessary for thyroid remnant ablation in low and intermediate risk patients? A pilot study with quality-of-life assessment. Brazilian Journal of Radiation Sciences. 2022;10(4):1–16. doi: 10.15392/2319-0612.2022.2047
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110
- Rosário PW, Vasconcelos FP, Cardoso LD, et al. Managing thyroid cancer without thyroxine withdrawal. Arquivos Brasileiros de Endocrinologia & Metabologia. 2006;50(1):91–96. doi: 10.1590/s0004-27302006000100013
- Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. The British Journal of Radiology. 1977;50(599):799–807. doi: 10.1259/0007-1285-50-599-799
- Giovanella L, Piccardo A. A “new/old method” for TSH stimulation: could a third way to prepare DTC patients for 131I remnant ablation possibly exist? European Journal of Nuclear Medicine and Molecular Imaging. 2015;43(2):221–223. doi: 10.1007/s00259-015-3245-9
- Semenov DYu, Boriskova ME, Farafonova UV, et al. Prognostic value of Sodium-Iodide Symporter (NIS) in differentiated thyroid cancer. Clinical and experimental thyroidology. 2015;11(1):50. (In Russ). doi: 10.14341/ket2015150-58
- Xiao J, Yun C, Cao J, et al. A pre-ablative thyroid-stimulating hormone with 30-70 mIU/L achieves better response to initial radioiodine remnant ablation in differentiated thyroid carcinoma patients. Scientific Reports. 2021;11(1). doi: 10.1038/s41598-020-80015-8
- Zhao T, Liang J, Guo Z, Li T, Lin Y. In Patients with Low- to Intermediate-Risk Thyroid Cancer, a Preablative Thyrotropin Level of 30 μIU/mL Is Not Adequate to Achieve Better Response to 131I Therapy. Clinical Nuclear Medicine. 2016;41(6):454–458. doi: 10.1097/rlu.0000000000001167
- Hasbek Z, Turgut B. Is Very High Thyroid Stimulating Hormone Level Required in Differentiated Thyroid Cancer for Ablation Success? Molecular Imaging and Radionuclide Therapy. 2016;25(2):79–84. doi: 10.4274/mirt.88598
- Vrachimis A, Riemann B, Mäder U, Reiners C, Verburg FA. Endogenous TSH levels at the time of 131I ablation do not influence ablation success, recurrence-free survival or differentiated thyroid cancer-related mortality. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43(2):224–231. doi: 10.1007/s00259-015-3223-2
- Montesano T, Durante C, Attard M, et al. Age influences TSH serum levels after withdrawal of l-thyroxine or rhTSH stimulation in patients affected by differentiated thyroid cancer. Biomedicine & Pharmacotherapy. 2007;61(8):468–471. doi: 10.1016/j.biopha.2007.04.001
- Ju N, Hou L, Song H, et al. TSH ≥30 mU/L may not be necessary for successful 131I remnant ablation in patients with differentiated thyroid cancer. European Thyroid Journal. 2023;12(4). doi: 10.1530/ETJ-22-0219
- Ren B, Zhu Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. International Journal of Molecular Sciences. 2022;23(5):2708. doi: 10.3390/ijms23052708
- Rubio GA, Catanuto P, Glassberg MK, Lew JI, Elliot SJ. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery. 2018;163(1):143–149. doi: 10.1016/j.surg.2017.04.031
- Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocrine-Related Cancer. 2014;21(5):T273–T283. doi: 10.1530/erc-14-0053
- Rajoria S, Suriano R, Shanmugam A, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20(1):33–41. doi: 10.1089/thy.2009.0296
- Tala H, Robbins R, Fagin JA, Larson SM, Tuttle RM. Five-year survival is similar thyroid cancer patients with metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):2105–2111. doi: 10.1210/jc.2011-0305
- Rosario PW, Mourão GF, Calsolari MR. Recombinant human TSH versus thyroid hormone withdrawal in adjuvant therapy with radioactive iodine of patients with papillary thyroid carcinoma and clinically apparent lymph node metastases not limited to the central compartment (cN1b). Archives of Endocrinology and Metabolism. 2017;61(2):167–172. doi: 10.1590/2359-3997000000247
- Hugo J, Robenshtok E, Grewal R, Larson S, Tuttle RM. Recombinant human thyroid stimulating hormone-assisted radioactive iodine remnant ablation in thyroid cancer patients at intermediate to high risk of recurrence. Thyroid. 2012;22(10):1007–1015. doi: 10.1089/thy.2012.0183
- Robenshtok E, Tuttle RM. Role of Recombinant Human Thyrotropin (rhTSH) in the Treatment of Well-Differentiated Thyroid Cancer. Indian Journal of Surgical Oncology. 2012;3(3):182–189. doi: 10.1007/s13193-011-0115-1
- Higuchi CRS, Fernanda P, Jurnior PA, et al. Clinical Outcomes After Radioiodine Therapy, According to the Method of Preparation by Recombinant TSH vs. Endogenous Hypothyroidism, in Thyroid Cancer Patients at Intermediate-High Risk of Recurrence. Frontiers in Nuclear Medicine. 2021;1. doi: 10.3389/fnume.2021.785768
- Lawhn-Heath C, Flavell RR, Chuang EY, Liu C. Failure of iodine uptake in microscopic pulmonary metastases after recombinant human thyroid-stimulating hormone stimulation. World Journal of Nuclear Medicine. 2020;19(1):61–64. doi: 10.4103/wjnm.WJNM_29_19
- Lee H, Paeng JC, Choi H, et al. Effect of TSH stimulation protocols on adequacy of low-iodine diet for radioiodine administration. PLoS One. 2021;16(9):e0256727. doi: 10.1371/journal.pone.0256727
- Driedger AA, Kotowycz N. Two Cases of Thyroid Carcinoma That Were Not Stimulated by Recombinant Human Thyrotropin. The Journal of Clinical Endocrinology & Metabolism. 2004;89(2):585–590. doi: 10.1210/jc.2003-031650
- Mernagh P, Campbell S, Dietlein M, et al. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: The German perspective. European Journal of Endocrinology. 2006;155(3):405–414. doi: 10.1530/eje.1.02223
- Mernagh P, Suebwongpat A, Silverberg J, Weston A. Cost-effectiveness of using recombinant human thyroid-stimulating hormone before radioiodine ablation for thyroid cancer: The Canadian perspective. Value in Health. 2010;13(3):180–187. doi: 10.1111/j.1524-4733.2009.00650.x
- Borget I, Bonastre J, Catargi B, et al. Quality of life and cost-effectiveness assessment of radioiodine ablation strategies in patients with thyroid cancer: results from the randomized phase III ESTIMABL trial. Journal of Clinical Oncology. 2015;33(26):2885–2892. doi: 10.1200/JCO.2015.61.6722
- Nijhuis TF, van Weperen W, de Klerk JMH. Costs associated with the withdrawal of thyroid hormone suppression therapy during the follow-up treatment of well-differentiated thyroid cancer. Tijdschrift voor nucleaire geneeskunde. 1999;21:98–100.
- Vallejo JA, Muros MA. Cost-effectiveness of using recombinant human thyroid-stimulating hormone before radioiodine ablation for thyroid cancer treatment in Spanish hospitals. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition). 2017;36(6):362–370. doi: 10.1016/j.remnie.2017.09.001
- Luster M, Felbinger R, Dietlein M, Reiners C. Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration. Thyroid. 2005;15(10):1147–1155. doi: 10.1089/thy.2005.15.1147
- Rosario PW, Xavier AC, Calsolari MR. Recombinant human thyrotropin in thyroid remnant ablation with 131I in high-risk patients. Thyroid. 2010;20(11):1247–1252. doi: 10.1089/thy.2010.0114
- Iizuka Y, Katagiri T, Ogura K, Inoue M, et al. Comparison of thyroid hormone withdrawal and recombinant human thyroid-stimulating hormone administration for adjuvant therapy in patients with intermediate- to high-risk differentiated thyroid cancer. Annals of Nuclear Medicine. 2020;34(10):736-741. doi: 10.1007/s12149-020-01497-0
- Robbins RJ, Driedger A, Magner J; The U.S. and Canadian Thyrogen Compassionate Use Program Investigator Group. Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid. 2006;16(11):1121–1130. doi: 10.1089/thy.2006.16.1121
- Tu J, Wang S, Huo Z, et al. Recombinant human thyrotropin-aided versus thyroid hormone withdrawal-aided radioiodine treatment for differentiated thyroid cancer after total thyroidectomy: a meta-analysis. Radiotherapy and Oncology. 2014;110(1):25–30. doi: 10.1016/j.radonc.2013.12.018
- Ma C, Xie J, Liu W, et al. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database of Systematic Reviews. 2010. doi: 10.1002/14651858.CD008302
- Wolfson RM, Rachinsky I, Morrison D, et al. Recombinant Human Thyroid Stimulating Hormone versus Thyroid Hormone Withdrawal for Radioactive Iodine Treatment of Differentiated Thyroid Cancer with Nodal Metastatic Disease. Journal of Oncology. 2016:1–6. doi: 10.1155/2016/6496750
- Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, et al. Treatment of metastatic thyroid cancer: relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid. 2012;22(3):310–317. doi: 10.1089/thy.2011.0235
- Wolffenbuttel BH, Coppes MH, Bongaerts AH, Glaudemans AW, Links TP. Unexpected symptoms after rhTSH administration due to occult thyroid carcinoma metastasis. The Netherlands journal of medicine. 2013;71(5):253–256.
- Tsai HC, Ho KC, Chen SH, et al. Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal. Diagnostics. 2022;12(1):221 doi: 10.3390/diagnostics12010221
- Goldberg LD, Ditchek NT. Thyroid carcinoma with spinal cord compression. JAMA: The Journal of the American Medical Association. 1981;245(9):953-954. doi: 10.1001/jama.1981.03310340043025
- Hoelting T, Tezelman S, Siperstein AE, Duh QY, Clark OH. Biphasic effects of thyrotropin on invasion and growth of papillary and follicular thyroid cancer in vitro. Thyroid. 1995;5(1):35–40. doi: 10.1089/thy.1995.5.35
- Pietz L, Michałek K, Waśko R, et al. Wpływ stymulacji endogennego TSH na wzrost resztkowej objetości tarczycy u chorych po całkowitej tyreoidektomii z powodu raka zróznicowanego tarczycy. Endokrynologia Polska. 2008;59:119–122. (In Polish)
- Dedov II, Rumyantsev PO, Nizhegorodova KS, et al. Recombinant human thyrotropin in radioiodine diagnostics and radioiodine ablation of patients with well-differentiated thyroid cancer: the first experience in Russia. Endocrine Surgery. 2018;12(3):128–139. (In Russ). doi: 10.14341/serg9806
- Saracyn M, Lubas A, Bober B, et al. Recombinant human thyrotropin worsens renal cortical perfusion and renal function in patients after total thyroidectomy due to differentiated thyroid cancer. Thyroid. 2020;30(5):653–660. doi: 10.1089/thy.2019.0372
- Chaker L, Razvi S, Bensenor IM, et al. Hypothyroidism. Nature Reviews Disease Primers. 2022;8(1). doi: 10.1038/s41572-022-00357-7
- Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nature Reviews Endocrinology. 2014;10(10):582–591. doi: 10.1038/nrendo.2014.143
- Lien EA, Nedrebo BG, Varhaug JE, et al. Plasma total homocysteine levels during short-term iatrogenic hypothyroidism. Journal of Clinical Endocrinology and Metabolism. 2000;85(3):1049–1053. doi: 10.1210/jcem.85.3.6439
- Bicikova M, Hampl R, Hill M, et al. Steroids, sex hormone-binding globulin, homocysteine, selected hormones and markers of lipid and carbohydrate metabolism in patients with severe hypothyroidism and their changes following thyroid hormone supplementation. Clinical Chemistry and Laboratory Medicine. 2003;41(3):284–292. doi: 10.1515/CCLM.2003.045
- Lee SJ, Lee HY, Lee WW, Kim SE. The effect of recombinant human thyroid stimulating hormone on sustaining liver and renal function in thyroid cancer patients during radioactive iodine therapy. Nuclear Medicine Communications. 2014;35(7):727–732. doi: 10.1097/MNM.0000000000000118
- Targher G, Montagnana M, Salvagno G, et al. Association between serum TSH, free T4 and serum liver enzyme activities in a large cohort of unselected outpatients. Clinical Endocrinology. 2008;68(3):481–484. doi: 10.1111/j.1365-2265.2007.03068.x
- Pearce EN, Wilson PW, Yang Q, Vasan RS, Braverman LE. Thyroid function and lipid subparticle sizes in patients with short-term hypothyroidism and a population-based cohort. The Journal of Clinical Endocrinology & Metabolism. 2008;93(3):888–894. doi: 10.1210/jc.2007-1987
- Ness GC, Lopez D, Chambers CM, et al. Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3- methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochemical Pharmacology. 1998;56(1):121–129. doi: 10.1016/S0006-2952(98)00119-1
- Pattaravimonporn N, Chaikijurajai T, Chamroonrat W, Sriphrapradang C. Myxedema Psychosis after Levothyroxine Withdrawal in Radioactive Iodine Treatment of Differentiated Thyroid Cancer: A Case Report. Case Reports in Oncology. 2021;14(3):1596–1600. doi: 10.1159/000520128
- Nagamachi S, Jinnouchi S, Nishii R, et al. Cerebral blood flow abnormalities induced by transient hypothyroidism after thyroidectomy – analysis by tc-99m-HMPAO and SPM96. Annals of Nuclear Medicine. 2004;18(6):469–477. doi: 10.1007/BF02984562
- Constant EL, De Volder AG, Ivanoiu A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. Journal of Clinical Endocrinology and Metabolism. 2001;86(8):3864–3870. doi: 10.1210/jcem.86.8.7749
- Duntas LH, Biondi B. Short-term hypothyroidism after Levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. European Journal of Endocrinology. 2007;156(1):13–19. doi: 10.1530/eje.1.02310
- Kao PF, Lin JD, Chiu CT, et al. Gastric emptying function changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Journal of Gastroenterology and Hepatology. 2004;19(6):655–660. doi: 10.1111/j.1440-1746.2003.03326.x
- Botella-Carretero JI, Prados A, Manzano L, et al. The effects of thyroid hormones on circulating markers of cell-mediated immune response, as studied in patients with differentiated thyroid carcinoma before and during thyroxine withdrawal. European Journal of Endocrinology. 2005;153(2):223–230. doi: 10.1530/eje.1.01951
- Duranton F, Lacoste A, Faurous P, et al. Exogenous thyrotropin improves renal function in euthyroid patients, while serum creatinine levels are increased in hypothyroidism. Clinical Kidney Journal. 2013;6(5):478–483. doi: 10.1093/ckj/sft092
- Coura-Filho GB, Willegaignon J, Buchpiguel CA, Sapienza MT. Effects of thyroid hormone withdrawal and recombinant human thyrotropin on glomerular filtration rate during radioiodine therapy for well-differentiated thyroid cancer. Thyroid. 2015;25(12):1291–1296. doi: 10.1089/thy.2015.0173
- An YS, Lee J, Kim HK, Lee SJ, Yoon JK. Effect of withdrawal of thyroid hormones versus administration of recombinant human thyroid-stimulating hormone on renal function in thyroid cancer patients. Scientific Reports. 2023;13(1). doi: 10.1038/s41598-023-27455-0
- Den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Correlation between severity of thyroid dysfunction and renal function. Clinical Endocrinology. 2005;62(4):423–427. doi: 10.1111/j.1365-2265.2005.02236.x
- Cho YY, Kim SK, Jung JH, et al. Long-term outcomes of renal function after radioactive iodine therapy for thyroid cancer according to preparation method: thyroid hormone withdrawal vs. recombinant human thyrotropin. Endocrine. 2019;64(2):293–298. doi: 10.1007/s12020-018-1807-x
- Kreisman SH, Hennessey JV. Consistent Reversible Elevations of Serum Creatinine Levels in Severe Hypothyroidism. Archives of Internal Medicine. 1999;159(1):79–82. doi: 10.1001/archinte.159.1.79
- Mariani LH, Berns JS. The Renal Manifestations of Thyroid Disease. Journal of the American Society of Nephrology. 2012;23(1):22–26. doi: 10.1681/ASN.2010070766
- Kim SK, Yun GY, Kim KH. et al. Severe hyponatremia following radioactive iodine therapy in patients with differentiated thyroid cancer. Thyroid. 2014;24(4):773–777. doi: 10.1089/thy.2013.0110
- Nozu T, Yoshida Y, Ohira M, Okumura T. Severe hyponatremia in association with I (131) therapy in a patient with metastatic thyroid cancer. Internal Medicine. 2011;50(19):2169–2174. doi: 10.2169/internalmedicine.50.5740
- Shakir MK, Krook LS, Schraml FV, Clyde PW. Symptomatic hyponatremia in association with a low-iodine diet and levothyroxine withdrawal prior to I131 in patients with metastatic thyroid carcinoma. Thyroid. 2008;18(7):787–792. doi: 10.1089/thy.2008.0050
- Al Nozha OM, Vautour L, How J. Life-threatening hyponatremia following a low-iodine diet: a case report and review of all reported cases. Endocrine Practice. 2011;17(5):e113–e117. doi: 10.4158/EP11045.CR
- Lee JE, Kim SK, Han KH, et al. Risk factors for developing hyponatremia in thyroid cancer patients undergoing radioactive iodine therapy. PLoS One. 2014;9(8):e106840. doi: 10.1371/journal.pone.0106840
- Horie I, Ando T, Imaizumi M, Usa T, Kawakami A. Hyperkalemia develops in some thyroidectomized patients undergoing thyroid hormone withdrawal in preparation for radioactive iodine ablation for thyroid carcinoma. Endocrine Practice. 2015;21(5):488–494. doi: 10.4158/EP14532.OR
- Hyer S, Kong A, Pratt B, Harmer C. Salivary gland toxicity after radioiodine therapy for thyroid cancer. Clinical Oncology. 2007;19(1):83–86. doi: 10.1016/j.clon.2006.11.005
- Riachy R, Ghazal N, Haidar MB, Elamine A, Nasrallah MP. Early Sialadenitis After Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Prevalence and Predictors. International Journal of Endocrinology. 2020;2020:1–7. doi: 10.1155/2020/8649794
- Adramerinas M, Andreadis D, Vahtsevanos K, Poulopoulos A, Pazaitou-Panayiotou K. Sialadenitis as a complication of radioiodine therapy in patients with thyroid cancer: where do we stand? Hormones. 2021;20(4):669–678. doi: 10.1007/s42000-021-00304-3
- Silberstein E. Prevention of radiation sialadenitis and glossitis after radioiodine-131 therapy of thyroid cancer. Journal of Nuclear Medicine. 2007;48 Suppl. 2.
- Ma C, Xie J, Jiang Z, Wang G, Zuo S. Does amifostine have radioprotective effects on salivary glands in high-dose radioactive iodine-treated differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(9):1778–1785. doi: 10.1007/s00259-009-1368-6
- Nakada K, Ishibashi T, Takei T. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? Journal of nuclear medicine. 2005;46(2):261–266.
- Le Roux MK, Graillon N, Guyot L. Salivary side effects after radioiodine treatment for differentiated papillary thyroid carcinoma: Long-term study. Head & Neck. 2020;42(11):3133–3140. doi: 10.1002/hed.26359
- Jentzen W, Balschuweit D, Schmitz J, et al. The influence of saliva flow stimulation on the absorbed radiation dose to the salivary glands during radioiodine therapy of thyroid cancer using (124) I PET(/CT) imaging. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(12):2298–2306. doi: 10.1007/s00259-010-1532-z
- Trukhin AA, Yartsev VD, Sheremeta MS, et al. Nasolacrimal Duct Obstruction Secondary to Radioactive Iodine-131 Therapy for Differentiated Thyroid Cancer. The Bulletin of the Scientific Centre for Expert Evaluation of Medicinal Products. Regulatory Research and Medicine Evaluation. 2022;12(4):415–424. doi: 10.30895/1991-2919-2022-12-4-415-424
- Iakovou I, Goulis DG, Tsinaslanidou Z,et al. Effect of recombinant human thyroid-stimulating hormone or levothyroxine withdrawal on salivary gland dysfunction after radioactive iodine administration for thyroid remnant ablation. Head & Neck. 2016;38 Suppl. 1:E227–230. doi: 10.1002/hed.23974
- Rosario PW, Calsolari MR. Salivary and lacrimal gland dysfunction after remnant ablation with radioactive iodine in patients with differentiated thyroid carcinoma prepared with recombinant human thyrotropin. Thyroid. 2013;23(5):617–619. doi: 10.1089/thy.2012.0050
- Molenaar RJ, Sidana S, Radivoyevitch T, et al. Risk of Hematologic Malignancies After Radioiodine Treatment of Well-Differentiated Thyroid Cancer. Journal of Clinical Oncology. 2018;36(18):1831–1839. doi: 10.1200/JCO.2017.75.0232
- Signore A, Campagna G, Marinaccio J, et al. Analysis of Short-Term and Stable DNA Damage in Patients with Differentiated Thyroid Cancer Treated with 131I in Hypothyroidism or with Recombinant Human Thyroid-Stimulating Hormone for Remnant Ablation. Journal of Nuclear Medicine. 2022;63(10):1515–1522. doi: 10.2967/jnumed.121.263442
- Sohn SY, Choi JY, Jang HW, et al. Association between excessive urinary iodine excretion and failure of radioactive iodine thyroid ablation in patients with papillary thyroid cancer. Thyroid. 2013;23(6):741–747. doi: 10.1089/thy.2012.0136
- Lakshmanan M, Schaffer A, Robbins J, Reynolds J, Norton J. A simplified low iodine diet in I-131 scanning and therapy of thyroid cancer. Clinical Nuclear Medicine. 1988;13(12):866–868. doi: 10.1097/00003072-198812000-00003
- Maxon HR, Thomas SR, Boehringer A, et al. Low iodine diet in I-131 ablation of thyroid remnants. Clinical Nuclear Medicine. 1983;8(3):123–126. doi: 10.1097/00003072-198303000-00006
- Tala Jury HP, Castagna MG, Fioravanti C, et al. Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. Journal of Clinical Endocrinology and Metabolism. 2010;95(1):230–237. doi: 10.1210/jc.2009-1624
- Lee M, Lee YK, Jeon TJ, et al. Low iodine diet for one week is sufficient for adequate preparation of high dose radioactive iodine ablation therapy of differentiated thyroid cancer patients in iodine-rich areas. Thyroid. 2014;24(8):1289–1296. doi: 10.1089/thy.2013.0695
- Tobey AE, Hongxiu L, Auh S, et al. Urine iodine excretion exceeding 250 ug/24h is associated with higher likelihood of progression in intermediate and high-risk thyroid cancer patients treated with radioactive iodine [abstract]. Thyroid. 2018;28 Suppl. 1:A40–A41
- Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clinical Endocrinology. 2003;58(4):428–435. doi: 10.1046/j.1365-2265.2003.01735.x
- Morris LF, Wilder MS, Waxman AD, Braunstein GD. Reevaluation of the impact of a stringent low-iodine diet on ablation rates in radioiodine treatment of thyroid carcinoma. Thyroid. 2001;11(8):749–755. doi: 10.1089/10507250152484583
- Yoo IDKS, Kim SH, Seo YY, et al. The success rate of initial (131) i ablation in differentiated thyroid cancer: comparison between less strict and very strict low iodine diets. Nuclear Medicine and Molecular Imaging. 2012;46(1):34–40. doi: 10.1007/s13139-011-0111-y
- Ito S, Iwano S, Kato K, Naganawa S. Predictive factors for the outcomes of initial I-131 low-dose ablation therapy to Japanese patients with differentiated thyroid cancer. journal article. Annals of Nuclear Medicine. 2018;32(6):418–424. doi: 10.1007/s12149-018-1261-0
- Lim CY, Kim JY, Yoon MJ, et al. Effect of a low iodine diet vs. restricted iodine diet on postsurgical preparation for radioiodine ablation therapy in thyroid carcinoma patients. Yonsei Medical Journal. 2015;56(4):1021–1027. doi: 10.3349/ymj.2015.56.4.1021
- Kim HK, Lee SY, Lee JI, et al. Daily urine iodine excretion while consuming a low-iodine diet in preparation for radioactive iodine therapy in a high iodine intake area. Clinical Endocrinology. 2011;75(6):851–856. doi: 10.1111/j.1365-2265.2011.04157.x
- Tomoda C, Uruno T, Takamura Y, et al. Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocrine Journal. 2005;52(2):237–240. doi: 10.1507/endocrj.52.237
- Sohaimi WF, Abdul Manap M, Kasilingam L, et al. Randomised controlled trial of one-week strict low-iodine diet versus one-week non-specified low iodine diet in differentiated thyroid carcinoma. Iranian Journal of Nuclear Medicine. 2019;27(2):99–105.
- Dekker BL, Links MH, Muller Kobold AC, et al. Low-Iodine Diet of 4 Days Is Sufficient Preparation for 131I Therapy in Differentiated Thyroid Cancer Patients. The Journal of Clinical Endocrinology & Metabolism. 2022;107(2):e604–e611. doi: 10.1210/clinem/dgab691
- Padovani RP, Maciel RM, Kasamatsu TS, et al. Assessment of the Effect of Two Distinct Restricted Iodine Diet Durations on Urinary Iodine Levels (Collected over 24 h or as a Single-Spot Urinary Sample) and Na (+)/I (-) Symporter Expression. European Thyroid Journal. 2015;4(2):99–105. doi: 10.1159/000433426
- Park JT, Hennessey JV. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 2004;14(1):57–63. doi: 10.1089/105072504322783858
- Dekker BL, Touw DJ, van der Horst-Schrivers ANA, et al. Use of Salivary Iodine Concentrations to Estimate the Iodine Status of Adults in Clinical Practice. The Journal of Nutrition. 2021;151(12):3671–3677. doi: 10.1093/jn/nxab303
Arquivos suplementares