Tissue sampling and histopathological limitations in esophageal cancer
- Autores: Akhmedzyanova D.A.1, Yutsevich O.K.2, Reshetnikov R.V.1, Tashchyаn O.V.3, Pirogov S.S.2, Mazurova M.P.2, Volchenko N.N.2, Kamalov A.K.2, Shumskaya Y.F.1, Mnatsakanyan M.G.3
-
Afiliações:
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
- The First Sechenov Moscow State Medical University
- Edição: Volume 4, Nº 4 (2023)
- Páginas: 633-642
- Seção: Case reports
- ##submission.dateSubmitted##: 18.07.2023
- ##submission.dateAccepted##: 16.11.2023
- ##submission.datePublished##: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/561354
- DOI: https://doi.org/10.17816/DD561354
- ID: 561354
Citar
Resumo
Esophageal adenocarcinoma is a common gastrointestinal cancer. Esophagogastroduodenoscopy with biopsy and immunohistochemistry are used to detect the neoplasm at an early stage. Definitive diagnosis requires not only highly specialized equipment but also the skills of the endoscopist and pathologist. We report the case of a 35-year-old man with progressive dysphagia caused by gastroesophageal cancer. Numerous esophagogastroduodenoscopy studies, computed tomography, and barium X-ray swallow revealed an extensive esophageal lesion; however, pathomorphologic examinations did not confirm malignancy within a year. Histological studies showed pyloric gland adenoma and adenoma from parietal or oncocytic cells with high-grade dysplasia. Esophagogastroduodenoscopy with targeted biopsy at a specialized center confirmed the tumor malignancy. This clinical case demonstrates the importance of summing clinical symptoms and using additional instrumental methods to make a definitive diagnosis if biopsy results are ambiguous.
Texto integral
BACKGROUND
Esophageal cancer is the sixth most common cause of cancer deaths worldwide [1], and the incidence of esophageal adenocarcinoma is rapidly increasing in developed countries [2]. This type of esophageal cancer is extremely aggressive, with a 5-year survival rate <20% [3]. Risk factors for esophageal adenocarcinoma and gastroesophageal junction adenocarcinoma include mechanical, chemical, and thermal injury to the esophageal mucosa, male sex, obesity, and smoking [4], with the most significant risk factor being Barrett’s esophagus caused by gastroesophageal reflux disease [5]. Prognosis is determined by both the tumor stage and its macroscopic morphology [6-8]. W.R.C. Knight et al. showed that ulcerating tumors were associated with a more favorable prognosis than exophytic or stenotic lesions [9].
Esophageal cancer is commonly diagnosed using radiological methods, specifically with double-contrast upper gastrointestinal radiography and computed tomography (CT) of the chest [10]. These methods allow evaluation of the lesion size, invasion depth, and tumor type. However, these findings must be verified by histopathology [3]. Esophagogastroduodenoscopy (EGD) and transnasal endoscopy are used to diagnose esophageal tumors. They allow visual examination of the organs for abnormalities and obtain biopsy specimens for morphological examination, which is considered the “gold standard” for tumor verification [11]. However, histological findings are dependent on various factors, including the qualifications and experience of the pathologist and endoscopist, quality of reagents used in specimen preservation, and quality of biopsy specimens obtained by endoscopic forceps biopsy. Therefore, biopsy results may not always match the clinical, endoscopic, or radiological findings [12]. Furthermore, diagnosing adenocarcinoma in the setting of Barrett’s esophagus is challenging because of its typically endophytic growth pattern, which necessitates a specific biopsy technique [13].
Herein, a case of a patient with progressive dysphagia who was diagnosed with esophageal adenocarcinoma that had spread to the middle third of the esophagus and subcardial stomach is presented. Clinical and instrumental findings were unambiguously indicative of malignancy. However, repeated morphological examinations did not confirm the diagnosis. A definitive diagnosis was made after an EGD-guided targeted biopsy at a tertiary cancer center.
This case report was prepared in accordance with the CARE Case Report Guidelines [14].
CASE REPORT
Medical History
Patient N was a 35-year-old man.
In March 2021, he experienced a gradual loss of appetite. He did not seek medical advice because he believed that the symptoms were caused by work-related stress.
In March 2022, he presented to his primary care physician with symptoms of difficulty swallowing, nausea and vomiting, and a throat lump sensation. At presentation, he was 192 cm tall and weighed 185 kg (body mass index, 50.18 kg/m2, class III obesity).
EGD revealed a tumor mass with multiple ulcerations in the esophagus, beginning 30 cm from the incisors and nearly extending to the cardia. No reliable pathological evidence of tumor cells, dysplastic cells, or atypical cells was found in the biopsy specimens.
In April 2022, the patient visited a gastroenterologist and underwent EGD at follow-up. The examination revealed a significantly narrowed lumen in the middle and lower thirds of the esophagus, which was caused by a circumferential tumor with focal destruction. The tumor’s proximal and distal edges were visualized at 25 and 45 cm from the incisors, respectively. Submucosal tumor infiltration was observed in the subcardial stomach. Repeated biopsy of the ulcerations demonstrated fragments of a villous tumor lined with columnar epithelium with low-grade dysplasia. Another EGD-guided biopsy revealed no signs of atypical cells.
Preliminary diagnosis. The observed pathological changes were consistent with the presentation of a pyloric gland adenoma with focal high-grade dysplasia arising from parietal or oncocytic cells that were highly suspicious of malignancy. Histological findings were inconsistent with clinical presentation and endoscopy. To address the inconsistencies between the endoscopic and histological findings, the tumor immunophenotype was examined by immunohistochemistry, which correlated with the pyloric tumor expressing gastric superficial–foveolar epithelial mucins and pyloric gland mucins. No p53 mutation was detected in the tumor cells, which did not exhibit high proliferative activity. Because the malignant nature of the tumor could not be conclusively established through immunohistochemistry, the mass was considered a pyloric gland adenoma. A local gastroenterologist suggested a dynamic follow-up.
In May 2022, the patient presented to a gastroenterologist with worsening symptoms of impaired food passage from the mouth to the stomach and a weight loss of 15 kg over the past 3 months (body mass index, 46.12 kg/m2). The patient was admitted to the gastroenterology department for examination and diagnosis verification.
Laboratory Data
Blood chemistry revealed hyperuricemia (uric acid, 634.8 mmol/L; normal range, 154–357 mmol/L) and high levels of nonspecific inflammatory markers:
- Erythrocyte sedimentation rate: elevation to 50 (normal range, 2–20) mm/h
- Fibrinogen: elevation to 4.81 (normal range, 1.8–4) g/L
- C-reactive protein: elevation to 11.4 (normal range, 0–5) mg/L.
Latent iron deficiency was also found:
- Hemoglobin: 147 (normal range, 132–180) g/L
- Color index: 0.9
- Iron: 5.9 (normal range, 10.7–32.2) mmol/L
Imaging Studies
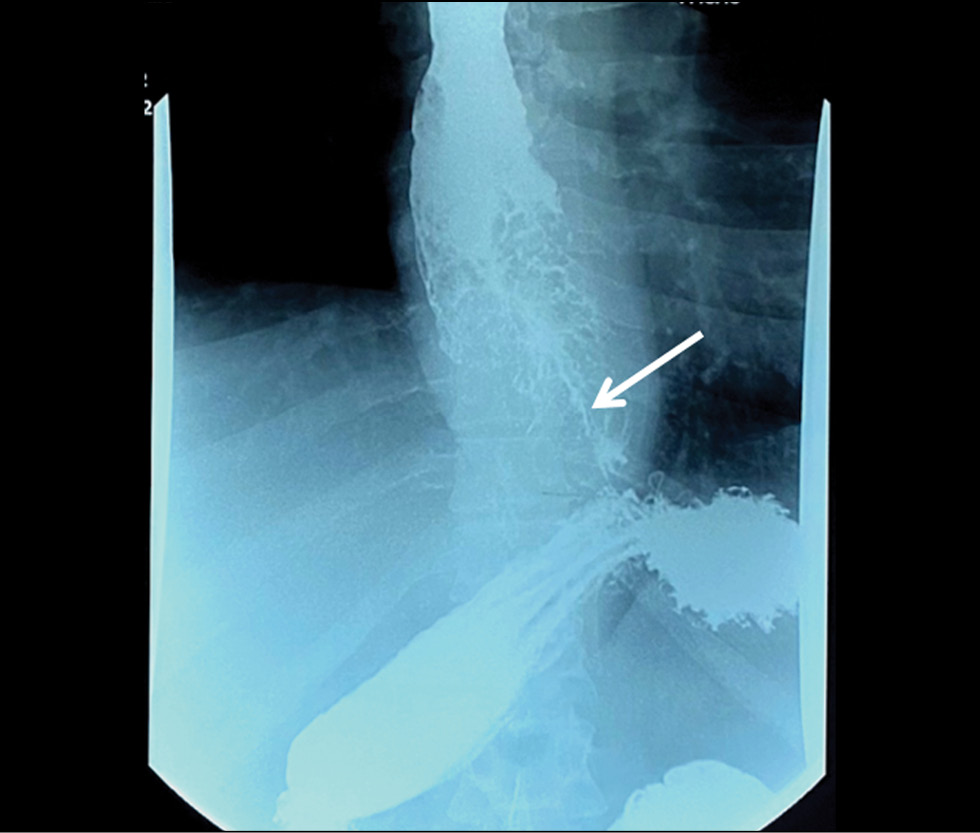
Barium-contrast upper gastrointestinal X-ray findings were suggestive of an extended mass lesion in the middle and lower thirds of the esophagus with luminal stenosis (Fig. 1).
Fig. 1. X-ray of the esophagus. Circular narrowing of the esophagus, a thin channel for the entry of barium suspension (indicated by an arrow).
Chest CT showed a 186-mm esophageal tumor that had extended to the cardia. The esophageal walls had a polypoid thickening of up to 41 mm, and a significant narrowing of the esophageal lumen to 2 mm was observed. Signs of regional lymph node involvement were also visible (Fig. 2).
Fig. 2. Computed tomography of the chest organs. White arrows indicate pathological changes: a — neoplasm of the esophagus, spreading to the cardia of the stomach, 186 mm long; b - massive proliferation of tumor tissue with narrowing of the lumen in the middle third of the esophagus; c — tumor lesion of the regional para-aortic lymph node; d — narrowing of the lumen of the esophagus to 2 mm in the lower third.
Abdominal CT did not reveal any distant metastases. The results demonstrated a locally advanced malignant esophageal tumor involving the cardia.
The patient was referred to a tertiary cancer center. Endoscopy at the cancer center showed the proximal edge of esophageal tumor infiltration at a site 24 cm from the incisors. The tumor appeared as multiple whitish-red merging lesions that spread circumferentially to the subcardial stomach. Deep ulcers covered with fibrin and necrotic plaque were observed. A fistula opening was found in the tumor tissue at a site 36 cm from the incisors, with the creamy opalescent contents flowing into the lumen. The esophageal lumen was significantly narrowed by the exophytic component of the tumor (Fig. 3). The tumor tissue was dough-like in texture and bled easily upon contact. The circumferentially infiltrated cardioesophageal junction was visualized at a site 44 cm from the incisors. The tumor infiltrated along the posterior wall to the subcardia (Fig. 4).
Fig. 3. Endophoto. Stenosing adenocarcinoma associated with Barrett`s esophagus. Blue arrows indicate areas of circularly located tumor.
Fig. 4. Endophoto. Barrett`s stenosing adenocarcinoma. The yellow arrow marks the endoscope located at the entrance to the stomach, the green arrow marks the tumor tissue.
Targeted stepwise biopsy of the non-necrotic regions was performed. The pathology results indicated low-grade esophageal adenocarcinoma progressing from Barrett’s esophagus.
Diagnosis and Treatment
The patient was diagnosed with low-grade cT3N1M0 adenocarcinoma progressing from Barrett’s esophagus. The tumor had spread to the cardia and was complicated by an esophageal–mediastinal fistula.
Given the patient’s young age and absence of long-term tumor metastasis, radical surgical treatment was deemed appropriate. In July 2022, the patient underwent single-step surgery including thoracoscopic esophageal resection, esophageal repair with a pedicle flap composed of a segment of the greater curvature of the stomach, cervical anastomosis formation, and 2S lymphadenectomy. During the surgical intervention, an esophageal–mediastinal fistula was also removed.
Pathological examination of the surgical specimen confirmed a low-grade esophageal adenocarcinoma progressing from Barrett’s esophagus with necrotic sites and surface ulceration. The tumor had infiltrated the mucous membrane and the submucosal muscle layer of the esophageal wall and had spread to the cardia. Tumor metastases were detected in 4 of 11 esophageal lymph nodes and 4 of 6 lymph nodes along the lesser curvature of the stomach.
The final diagnosis was stage III pT4N1M0 esophageal adenocarcinoma progressing from Barrett’s esophagus.
The postoperative period was complicated by a tracheoesophageal fistula, which was epithelialized after 3 weeks of endoscopic vacuum therapy.Given the advanced stage of the primary tumor, the high risk of disease recurrence, and young patient age, he received nine cycles of adjuvant FOLFOX (calcium folinate, fluorouracil, and oxaliplatin).
Follow-up examinations in December 2022 and April 2023 showed no signs of local tumor recurrence or progression.
DISCUSSION
This clinical case report discloses several problems associated with the endoscopic and pathological diagnosis of a malignant tumor progressing from Barrett’s esophagus. Histological confirmation of the tumor process and its type is vital before surgery, or other treatments can be performed. The decision to use chemotherapy or combined chemoradiotherapy, either as an adjuvant or neoadjuvant treatment, depends on both the tumor stage and its histological pattern. In this case, the main issue was the inconsistency between the endoscopy and radiology results and the histological findings.
Advanced endoscopic equipment provides high-resolution imaging of the mucous membrane of hollow organs. The primary principle should be to perform a comprehensive examination of the entire organ and identify the most suspicious regions of the mucous membrane. This is a more labor-intensive and time-consuming manipulation. The decision to perform a targeted biopsy should only be made after a detailed endoscopy. Targeted biopsy differs from blind biopsy and classic forceps biopsy by taking specimens from the most suspicious regions using more specific techniques such as narrowed-spectrum endoscopy combined with near-focus mode [15].
Early-stage adenocarcinoma progressing from Barrett’s esophagus is most commonly characterized by a flat growth pattern within the metaplasia segment [16]. In our case, the esophageal carcinoma was accompanied by massive adenomatous tissue growth, which delayed the malignancy verification because adenomatous tissue samples were taken in multiple nontargeted biopsies. The diagnosis was established accurately through a targeted, stepwise biopsy of the non-necrotic regions.
A study reported that only 35% of patients experience correct detection and preoperative staging of esophageal adenocarcinomas [17], a finding supported by this case. One reason for this is the insufficient accuracy of forceps biopsy as a means of obtaining pathology specimens. If esophageal malignancy is suspected, endoscopic mucosal resection is warranted [18]. The value of a biopsy is increased when at least five tissue fragments are obtained. This increases the likelihood of detecting atypical tumor cells, even incidentally, in one of the fragments [19, 20].
Pathologists may disagree on whether the changes detected in the biopsy specimens are caused by dysplasia or signs of a malignant tumor. According to A.H. Ormsby et al., pathologists specializing in gastrointestinal tissues frequently disagree on the diagnosis of high-grade dysplasia versus adenocarcinoma, even when evaluating total resection specimens [21]. The authors suggested revising treatment strategies that differentiate between severe dysplasia and intramucosal adenocarcinoma based on histological differences using a limited number of biopsies.
CONCLUSION
This case report demonstrates the significance of a clinician’s critical approach to pathology results. The diagnosis should also be based on the clinical presentation and instrumental findings. However, in cases of unclear histological findings, biopsy samples from the tumor sites with the highest quality or volume are recommended, even if multiple biopsies are required.
ADDITIONAL INFORMATION
Funding source. This article was prepared by a group of authors as a part of the research and development effort titled “Opportunistic screening of high-profile and other common diseases”, No. 123031400009-1”, (USIS No. 123031400009-1) in accordance with the Order No. 1196 dated December 21, 2022 “On approval of state assignments funded by means of allocations from the budget of the city of Moscow to the state budgetary (autonomous) institutions subordinate to the Moscow Health Care Department, for 2023 and the planned period of 2024 and 2025” issued by the Moscow Health Care Department.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. D.A. Akhmedzyanova — concept, collection and processing of data, data analysis, manuscript writing; O.K. Yutsevich — collection and processing of data, manuscript writing; R.V. Reshetnikov — concept, manuscript editing; O.V. Tashchyan, S.S. Pirogov, M.P. Mazurova, N.N. Volchenko, A.K. Kamalov, Y.F. Shumskaya — manuscript editing, preparation of illustrative material; M.G. Mnatsakanyan — final editing, manuscript approval.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics Journal.
Acknowledgments. The authors express their gratitude to Ivan A. Blokhin for his support in the text editing.
Sobre autores
Dina Akhmedzyanova
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Autor responsável pela correspondência
Email: AkhmedzyanovaDA@zdrav.mos.ru
ORCID ID: 0000-0001-7705-9754
Código SPIN: 6983-5991
Scopus Author ID: 58104960900
Rússia, Moscow
Olga Yutsevich
P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
Email: o.yutsevitch@yandex.ru
ORCID ID: 0000-0002-3860-9853
Rússia, Moscow
Roman Reshetnikov
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: r.reshetnikov@npcmr.ru
ORCID ID: 0000-0002-9661-0254
Código SPIN: 8592-0558
Cand. Sci. (Phys.-Math.)
Rússia, MoscowOlga Tashchyаn
The First Sechenov Moscow State Medical University
Email: olgatash1@rambler.ru
ORCID ID: 0000-0001-6759-6820
Código SPIN: 3658-1120
MD, Cand. Sci. (Med.)
Rússia, MoscowSergey Pirogov
P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
Email: pirogov@mail.ru
ORCID ID: 0000-0002-8101-2155
Código SPIN: 7812-5502
MD, Dr. Sci. (Med.)
Rússia, MoscowMaria Mazurova
P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
Email: mnioi_morphology@mail.ru
ORCID ID: 0000-0002-4873-4455
Código SPIN: 4455-3055
MD, Cand. Sci. (Med.)
Rússia, MoscowNadezhda Volchenko
P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
Email: mnioi_morphology@mail.ru
ORCID ID: 0000-0003-0421-4172
MD, Dr. Sci. (Med.), Professor
Rússia, MoscowAziz Kamalov
P.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Research Radiological Center
Email: kak6768@mail.ru
ORCID ID: 0000-0001-7376-6056
Código SPIN: 1671-1600
Rússia, Moscow
Yuliya Shumskaya
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: ShumskayaYF@zdrav.mos.ru
ORCID ID: 0000-0002-8521-4045
Código SPIN: 3164-5518
Rússia, Moscow
Marina Mnatsakanyan
The First Sechenov Moscow State Medical University
Email: mnatsakanyan08@mail.ru
ORCID ID: 0000-0001-9337-7453
Código SPIN: 2015-1822
MD, Dr. Sci. (Med.), Professor
Rússia, MoscowBibliografia
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492
- McColl KEL. What is causing the rising incidence of esophageal adenocarcinoma in the West and will it also happen in the East? J Gastroenterol. 2019;54(8):669–673. doi: 10.1007/s00535-019-01593-7
- Joseph A, Raja S, Kamath S, et al. Esophageal adenocarcinoma: A dire need for early detection and treatment. Cleve Clin J Med. 2022;89(5):269–279. doi: 10.3949/ccjm.89a.21053
- Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi: 10.1007/s12328-020-01237-x
- Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett. 2009;275(2):170–177. doi: 10.1016/j.canlet.2008.07.006
- Deng HY, Alai G, Luo J, et al. Cancerous esophageal stenosis before treatment was significantly correlated to poor prognosis of patients with esophageal cancer: a meta-analysis. J Thorac Dis. 2018;10(7):4212–4219. doi: 10.21037/jtd.2018.06.89
- Sillah K, Pritchard SA, Watkins GR, et al. The degree of circumferential tumour involvement as a prognostic factor in oesophageal cancer. Eur J Cardiothorac Surg. 2009;36(2):368–373. doi: 10.1016/j.ejcts.2008.12.052
- Deng HY, Li G, Luo J. Does oesophageal stenosis have any impact on survival of oesophageal cancer patients? Interact Cardiovasc Thorac Surg. 2018;27(3):384–386. doi: 10.1093/icvts/ivy095
- Knight WRC, McEwen R, Byrne BE, et al. Endoscopic tumour morphology impacts survival in adenocarcinoma of the oesophagus. Eur J Surg Oncol. 2020;46(12):2257–2261. doi: 10.1016/j.ejso.2020.07.003
- Morozov SP, editor. I-74 Informativeness of radial diagnostics methods in various pathological conditions of the organism. Section 2: Diagnostics of pathological conditions and diseases of the gastrointestinal tract. Moscow; 2018. (In Russ).
- Ishihara R, Goda K, Oyama T. Endoscopic diagnosis and treatment of esophageal adenocarcinoma: introduction of Japan Esophageal Society classification of Barrett’s esophagus. J Gastroenterol. 2019;54(1):1–9. doi: 10.1007/s00535-018-1491-x
- Zagajnova EV, Zagajnov VE, Gladkova ND, et al. Optical coherence tomography in surgical treatment of esophageal cancer. Grekov’s Bulletin of Surgery. 2007;166(2):22–26.
- Davydov MI, Ter-Ovanesov MD, Stilidi IS, et al. Barrett’s esophagus: from theoretical foundations to practical recommendations. Practical oncology. 2003;4(2):109–119. (In Russ).
- Barber MS, Aronson JK, von Schoen-Angerer T, et al. CARe guidelines for case reports: explanation and elaboration document. Translation into Russian. Digital Diagnostics. 2022;3(1):16–42. doi: 10.17816/DD105291
- Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151(5):822–835. doi: 10.1053/j.gastro.2016.09.040
- di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology. 2018;154(2):421–436. doi: 10.1053/j.gastro.2017.07.041
- Winiker M, Mantziari S, Figueiredo SG, et al. Accuracy of preoperative staging for a priori resectable esophageal cancer. Dis Esophagus. 2018;31(1):1–6. doi: 10.1093/dote/dox113
- Elsadek HM, Radwan MM. Diagnostic Accuracy of Mucosal Biopsy versus Endoscopic Mucosal Resection in Barrett’s Esophagus and Related Superficial Lesions. Int Sch Res Notices. 2015;2015. doi: 10.1155/2015/735807
- Tryakin AA, Besova NS, Volkov NM, et al. Practice guidelines for drug treatment of esophageal and gastroesophageal junction cancers. Malignant tumours (Zlokačestvennye opuholi). 2021;11(3S2-1):299–313. (In Russ). doi: 10.18027/2224-5057-2021-11-3s2-20
- Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(4):393–422. doi: 10.6004/jnccn.2023.0019
- Ormsby AH, Petras RE, Henricks WH, et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51(5):671–676. doi: 10.1136/gut.51.5.671
Arquivos suplementares