Объективные критерии МРТ-оценки эффективности лечения метастазов в кости у больных раком предстательной железы и раком молочной железы: систематический обзор и метаанализ
- Авторы: Рипп В.О.1, Березовская Т.П.1, Иванов С.А.1
-
Учреждения:
- Медицинский радиологический научный центр имени А.Ф. Цыба — филиал федерального государственного бюджетного учреждения, «Национальный медицинский исследовательский центр радиологии» Министерства здравоохранения Российской Федерации
- Выпуск: Том 2, № 3 (2021)
- Страницы: 289-300
- Раздел: Систематические обзоры
- Статья получена: 03.08.2021
- Статья одобрена: 02.09.2021
- Статья опубликована: 15.10.2021
- URL: https://jdigitaldiagnostics.com/DD/article/view/77311
- DOI: https://doi.org/10.17816/DD77311
- ID: 77311
Цитировать
Аннотация
Обоснование. Возможность персонифицированного подхода к лечению метастатического рака предстательной железы (РПЖ) и рака молочной железы (РМЖ) требует объективных методов оценки ответа на лечение очагов в скелете. Доказанная высокая эффективность МРТ в выявлении метастазов в кости в сочетании с отсутствием ионизирующего излучения создаёт предпосылки для использования метода в мониторировании хода лечения на основе объективных критериев оценки терапевтического эффекта.
Цель ― оценить возможности объективных количественных и полуколичественных МРТ-критериев в определении эффективности лечения (радио-, химио-, гормоно- и таргетная терапия) метастазов в кости, применявшихся в клинических исследованиях у больных РПЖ и РМЖ.
Материалы и методы. Поиск в базах данных Embase, PubMed, Cochrane Central Register of Controlled trials (CENTRAL), eLibrary осуществлялся до 01.06.2021 по ключевым словам «magnetic resonance imaging», «MRI», «DWI», «treatment response», «prostate cancer», «breast cancer», «bone metastasis» на английском и русском языках. В обзор включали только исследования по объективной МРТ-оценке эффективности любого типа лечебного воздействия (за исключением хирургии) при метастатическом поражении скелета.
Результаты. На основании анализа 11 исследований, отобранных из 312 источников, выделены 4 группы объективных МРТ-критериев оценки терапевтического эффекта при метастатическом поражении костей у больных РПЖ и РМЖ, включающих динамику размеров, интенсивности сигнала на диффузионно-взвешенном изображении, числовых значений измеряемого коэффициента диффузии (ИКД), общей опухолевой нагрузки. Изменения этих количественных и полуколичественных показателей во всех работах, за единственным исключением, имели одинаковую направленность, хотя и различались числовыми значениями. Учитывая статистически значимую гетерогенность (p <0,1 для критерия χ2 и при I2 >40%) для значений ИКД до и после лечения, для анализа использовалась модель случайных эффектов. Изменение ИКД в результате лечения в среднем составило +0,35 [+0,12; +0,49] ×10-3 мм2/с со средними значениями ИКД до лечения 0,83 [0,71; 1,03] ×10-3 мм2/с, после лечения ― 1,18 [0,83; 1,49] ×10-3 мм2/с.
Заключение. МРТ является информативной методикой для объективной оценки ответа костных метастазов на терапию у больных РПЖ и РМЖ на основе количественных и полуколичественных критериев и имеет значительный потенциал в качестве диагностического инструмента для мониторирования эффективности лечения метастатического поражения скелета.
Полный текст
Список сокращений
ВВЕДЕНИЕ
Метастатическое поражение скелета часто встречается при диссеминации рака предстательной железы (РПЖ) и рака молочной железы (РМЖ), обнаруживается в 70–80% случаев аутопсий, иногда являясь единственной локализацией отдалённых метастазов. Более чем у 50% пациентов отдалённое метастазирование начинается с поражения костей [1–3].
Появление костных метастазов приводит к ухудшению физического, функционального, эмоционального состояния больных и сокращает продолжительность жизни. Если при терапии олигометастатического поражения возможно проведение радикального лечения, то при диссеминированном процессе прогноз значительно хуже, и лечение носит преимущественно паллиативный характер, направленный на улучшение общего качества жизни таких пациентов. Совершенствование химио- и гормонотерапии, разработка агрессивной мультимодальной терапии позволяют индивидуализировать лечение, корректируя его на основе оценки достигнутого терапевтического эффекта [4, 5].
Магнитно-резонансная томография (МРТ) зарекомендовала себя эффективным методом в выявлении метастатического поражения скелета с более высокими показателями чувствительности и специфичности (90,5 и 95% соответственно), чем сцинтиграфия (72,9 и 93,9%), и по крайней мере сопоставимыми ― с совмещённой позитронно-эмиссионной и компьютерной томографией (ПЭТ-КТ) с холином (89,7 и 96%) [6–8]. Возможность использования МРТ для оценки эффективности лечения метастазов в кости начали изучать достаточно давно, используя динамику размеров и интенсивности МР-сигнала на конвенциальных изображениях. С появлением диффузионно-взвешенного изображения (ДВИ) и МРТ всего тела появились новые критерии оценки ответа на лечение очагов в костях. Однако до настоящего времени не выработано единого подхода к МРТ-оценке эффективности лечения метастазов в кости на основе объективных критериев, а данные о МРТ-семиотике ответивших очагов противоречивы [9–12].
Цель исследования ― оценить возможности объективных количественных и полуколичественных МРТ-критериев в определении эффективности лечения (радиотерапия, химио- и гормонотерапия, таргетная терапия) метастазов в кости, применявшихся в клинических исследованиях у больных РПЖ и РМЖ.
МЕТОДЫ
Данная работа написана в соответствии с критериями PRISMA (предпочтительные параметры отчётности для систематических обзоров и метаанализа) [13].
Критерии соответствия
Критерии включения. Подходящие публикации выбирали по принципу PICOS [14]. Пациенты (P): от 18 лет и старше, имеющие гистологически подтверждённый РМЖ или РПЖ с метастатическим поражением костей. Тип вмешательства (I): МРТ как отдельных областей, так и всего тела; в протокол МРТ должны входить стандартные анатомические импульсные последовательности (Т1-, Т2-взвешенные изображения, STIR) и/или ДВИ с построением карт измеряемого коэффициента диффузии (ИКД). Группа сравнения (C): нет. Исходы (O): сканирование костных метастазов проводили не ранее чем за неделю до начала и через 1–6 мес от начала противоопухолевого лечения; эталонная оценка ответа для его сопоставления с данными МРТ проводилась с помощью методов лабораторной диагностики (определение уровня простатспецифического антигена в крови), результатов инструментальных исследований (спиральная компьютерная томография, сцинтиграфия скелета и ПЭТ-КТ), а также гистологического исследования с оценкой лечебного патоморфоза опухоли. Типы исследований (S): работы, в которых проводилось МРТ до начала и спустя 1–6 мес от начала противоопухолевого лечения. Ограничения по типу получаемой пациентами терапии (химическая, гормональная, таргетная, лучевая) отсутствовали.
Критерии невключения: пациенты, получившие хирургическое лечение метастатических очагов в костях.
Принимая во внимание технические разработки в области МРТ, были отобраны исследования, включающие:
1) оценку ДВИ и ИКД, опубликованные после 1 января 2010 г.;
2) только стандартные анатомические последовательности, опубликованные после 1 января 1998 г.
Критерии исключения: статьи, написанные не на русском и английском языках; тезисы конференций, описание клинических случаев; исследования на животных.
Источники информации
Поиск и отбор публикаций проводили в электронных базах данных Embase, PubMed, Cochrane Central Register of Controlled trials (CENTRAL), eLibrary. Последний поиск осуществлён 01 июня 2021 г.
Поиск. Поиск проводился среди проспективных и ретроспективных клинических исследований и рандомизированных контролируемых исследований c использованием следующих терминов библиотеки MeSH и ключевых слов: «Magnetic resonance imaging», «DWI», «treatment», «response», «prostate cancer», «breast cancer», «bone metastasis».
Отбор исследований. Поиск и последующий отбор проводился двумя независимыми рецензентами. Любые разногласия и вопросы, требующие обсуждения, разрешались главным исследователем. На первом этапе рассматривались названия/тезисы/абстракты на предмет соответствия теме обзора и возможности наличия необходимых данных. На втором этапе проводился полный анализ публикаций в соответствии с критериями включения и исключения.
Сбор данных. Для сбора данных была разработана форма-таблица. Двумя рецензентами была извлечена следующая информация: название публикации, год публикации, авторы, DOI, первичный очаг, популяция пациентов, метод лечения, сроки исследования, используемые импульсные последовательности, референсный метод, МР-семиотика, значения ИКД и интенсивности сигнала до лечения, МР-семиотика, значения ИКД и интенсивности сигнала после лечения.
Риск систематической ошибки. Для оценки риска систематической ошибки и предвзятости в отдельных исследованиях использован контрольный список вопросника QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) [15]. Тексты публикаций оценивались по следующим критериям: набор пациентов, исследуемая методика, эталонная методика, временные интервалы между исследуемой и эталонной методикой. Отдельные вопросы из списка QUADAS-2 не использовали ввиду отсутствия необходимости (например, в работах, где оценивали ИКД, не использовали вопрос, связанный с ослеплением интерпретатора исследования, так как ИКД ― это объективная оценка). После анализа каждого исследования двумя рецензентами на предмет риска систематической ошибки была составлена гистограмма.
Статистический анализ
Выбор метода анализа и группировки данных (модель случайных эффектов или модель фиксированного эффекта) осуществляли по результатам оценки гетерогенности исследований, включавших определение ИКД, которая проводилась с использованием критерия χ2 и индекса гетерогенности I2. Гетерогенность исследований считали статистически значимой при p <0,1 для критерия χ2 и при I2 >40%.
РЕЗУЛЬТАТЫ
Отбор исследований
Из 312 источников, найденных в базах данных Embase, PubMed, Cochrane Central Register of Controlled trials (CENTRAL), eLibrary, в настоящее исследование были включены 11 исследований (рис. 1).
Рис. 1. Блок-схема PRISMA данного исследования
На первом этапе после отсеивания дубликатов (31) и исследований на животных (8) осталось 273 источника. Далее, на этапе скрининга, после оценки абстрактов и тезисов была отсеяна 181 публикация. После проверки полнотекстовых вариантов работ на предмет соответствия критериям включения осталось 11 источников, которые были включены в настоящий обзор (таблица).
Таблица. Характеристика исследований, включённых в систематический обзор и метаанализ
№ | Авторы | Год | Нозология | Вид лечения | Интервал между исследованиями | Индукция магнитного поля, Тл | Модель, производитель МРТ | Объективные критерии |
1 | Byun и соавт. [5] | 2002 | РМЖ (90%) | РТ | 6 мес | 1,5 | Не указан | ИКД, ИС (ДВИ) |
2 | Messiou и соавт. [9] | 2011 | РПЖ | ХТ | 3 мес | 1,5 | Avanto, Siemens | ИКД |
3 | Reischauer и соавт. [10] | 2010 | РПЖ | ГТ | 1–3 мес | 1,5 | Achieva, Philips | ИКД |
4 | Perez-Lopez и соавт. [11] | 2016 | РПЖ | TT | 3 мес | 1,5 | Avanto, Siemens | ИКД, tDV |
5 | Blackledge и соавт. [12] | 2014 | РПЖ, РМЖ | ХТ, ГТ, РТ, ТТ | 10–38 нед. | 1,5 | Avanto, Aera, Siemens | ИКД, tDV |
6 | Сергеев и соавт. [16] | 2016 | РМЖ, РПЖ (90%) | ХТ, ГТ, РТ | 2–8 мес | 1,5 | Excelart, Toshiba | ИКД, ИС (ДВИ) |
7 | Çiray и соавт. [17] | 2001 | РМЖ | ХТ, ГТ | 3–6 мес | 0,5 | Gyroscan T5, Philips | Размер |
8 | Brown и соавт. [18] | 1998 | РМЖ | ГТ, ХТ, РТ | 6; 9 мес | 1,5 | Vision, Siemens | Размер |
9 | Tombal и соавт. [19] | 2005 | РПЖ | ХТ | 6 мес | 1,5 | Intera, Philips | Размер |
10 | Cappabianca и соавт. [20] | 2014 | РПЖ, РМЖ (90%) | РТ | 1; 2 мес | 1,5 | Symphony, Siemens, | ИКД, ИС (ДВИ) |
11 | Котлярови соавт. [21] | 2006 | РМЖ, РПЖ (75%) | ХТ, ГТ, РТ | 2–8 мес | 0,5 | Proview Open, Philips | Размер |
Примечание. МРТ ― магнитно-резонансная томография; РМЖ ― рак молочной железы; РПЖ ― рак предстательной железы; ХТ ― химиотерапия; ГТ ― гормонотерапия; РТ ― радиотерапия; ТТ ― таргетная терапия; ИКД ― измеряемый коэффициент диффузии; ИС ― интенсивность сигнала; ДВИ ― диффузионно-взвешенные изображения; tDV ― общая опухолевая нагрузка.
Всего включено 370 пациентов, из них 147 ответили на лечение. В трёх источниках [5, 16, 17] данные о количестве ответивших отсутствовали. Объём выборки каждого из включённых исследований варьировал от 10 до 87 пациентов. В семи исследованиях [9–12, 17–19] в выборку входили только пациенты с первичным очагом в предстательной или молочной железе, в четырёх работах [5, 16, 20, 21] эти локализации составили 85–95% для всей выборки. Средний возраст пациентов варьировал от 47 до 73 лет.
Публикации были разделены на 4 группы в соответствии с используемыми критериями оценки ответа на системную терапию:
- размеры метастатических очагов [17–19, 21];
- интенсивность сигнала на ДВИ [5, 16, 20];
- измеряемый коэффициент диффузии [5, 9–12, 16, 20];
- общий опухолевый объём [11, 12].
В шести работах [9–11, 16, 17, 20] в качестве эталонного теста использовали КТ, сцинтиграфию скелета, простатспецифический антиген (у больных раком предстательной железы), в двух ― кроме перечисленных методик применялись ПЭТ [12] и биопсия [5]. В трёх публикациях [18, 19, 21] данные об используемой эталонной методике не указаны.
Параметры сканирования
Параметры сканирования при использовании Т1-SE- последовательности в различных исследованиях имели незначительные отличия значений TR и TE (от 400 до 600 мс и от 5 до 16 мс соответственно). Только в одной публикации [10] параметры Т1-ВИ значительно отличались и составили 1000 и 3,7 мс. В пяти исследованиях [5, 10–12, 16] с применением ДВИ количество b-факторов составляло 2–3 с начальными и наиболее высокими значениями 0–50 и 800–900 соответственно. В одной работе [9] количество b-факторов составило 4 (0; 50; 250; 750), ещё в одной [20] ― 6 (0; 50; 100; 250; 500; 750), при этом в обеих получены более высокие значения ИКД в сравнении с остальными исследованиями, что может быть следствием изменения параметров сканирования. Толщина среза во всех исследованиях находилась в пределах 4–6 мм как для Т1-ВИ, так и для ДВИ.
Классификация объективных оценочных критериев, используемых в литературе
Первым появившимся критерием терапевтического ответа костных метастазов было изменение размеров очага. Стоит отметить, что измерение очагов в костях рекомендуется проводить на Т1-ВИ, где они более контрастны, имеют более чёткие контуры и менее выражен перифокальный отёк [6, 22]. Этот критерий был использован в четырёх из 11 отобранных нами работ.
Вторым критерием оценки ответа было полуколичественное определение интенсивности сигнала на ДВИ, встретившееся в 3 из 11 работ. Так как интенсивность сигнала не является физической величиной, её числовое значение может отличаться на разных МР-томографах и при разных параметрах сканирования, поэтому используют отношение интенсивности сигнала в метастазах к интенсивности сигнала в мышцах (signal intensity ratio, SIR).
Третьим критерием оценки ответа на лечение является изменение числового значения ИКД в опухолевых очагах, которое рассчитывают и картируют на основе ДВИ с несколькими значениями b-факторов. Этот критерий получил наибольшее распространение в последнее время и был использован в 7 работах из 11, однако следует отметить, что значения ИКД зависят от параметров изображения.
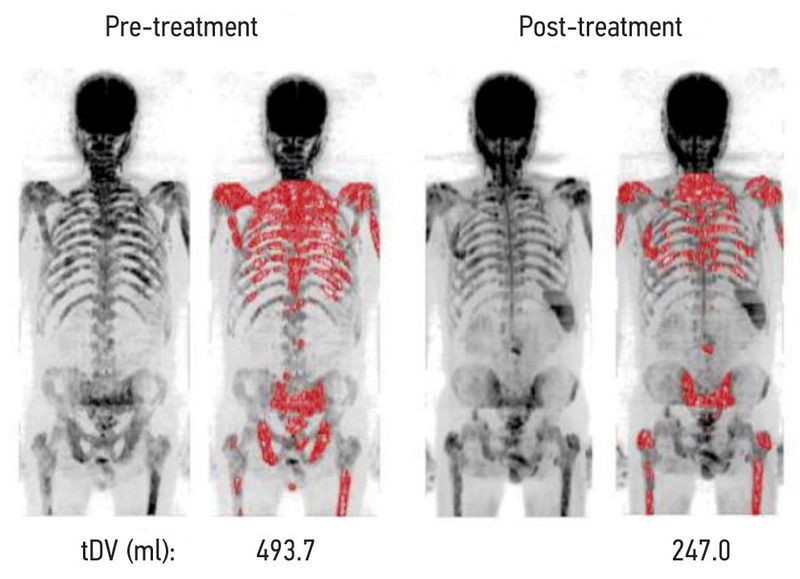
Рис. 2. Визуализация и значения общей опухолевой нагрузки до и после лечения у пациента с выраженным ответом на системную терапию [12].
Четвёртым, наиболее новым критерием является определение общей опухолевой нагрузки (total diffusion volume, tDV). Объём опухолевой нагрузки (в мл) автоматически рассчитывается по проекции максимальной интенсивности (maximum intensity projection, MIP) ДВИ с полуавтоматически построенной и вручную скорректированной 3D-маской путём подсчёта количества вокселей в заданном диапазоне интенсивности сигнала (рис. 2). Распределение количества вокселей по диапазонам ИКД, соответствующим ответившим, не ответившим и сомнительным очагам, может быть представлено в виде гистограммы [11, 12, 23].
Риск систематической ошибки
Имеется риск систематической ошибки за счёт различия в применяемых методах противоопухолевой терапии и отличий во временных интервалах между МРТ до и после начала лечения, хотя в 50% публикаций интервал составил 2–3 мес. Основным моментом риска систематической ошибки по критериям QUADAS-2 был подбор эталонной методики (рис. 3).
Рис. 3. Риск систематической ошибки по QUADAS-2.
В пяти публикациях в качестве эталонных выступали разные методики визуализации для разных пациентов [5, 12], либо это была только сцинтиграфия [16, 17, 20], которая уступает в диагностической ценности МРТ и не имеет необходимых значений чувствительности и специфичности, близких к 100%. В трёх публикациях эталонная методика отсутствовала вовсе [18, 19, 21], а оценку ответа на терапию контролировали по уровню простатспецифического антигена и клиническим данным. В двух работах [17, 18] исследуемая методика проводилась на МР-томографах с напряжённостью магнитного поля 0,5 Тл, что также могло стать источником систематической ошибки.
Результаты оценки ответа костных очагов на системную терапию
Размер. Изменение размеров на Т1-ВИ оценивалось в четырёх публикациях [17–19, 21], в трёх из которых отмечено значительное уменьшение как отдельно взятых ответивших очагов [18, 19, 21], так и общего опухолевого размера (tumor mass index, TMI), полученного в результате суммации размеров всех метастатических очагов в двух измерениях [17], а B. Tombal с соавт. [19] даже наблюдали полное исчезновение метастатических очагов в двух случаях. Однако стоит учитывать, что повторное исследование они проводили спустя 6 мес, а не через 2–3 мес, как в остальных публикациях. В работе A.L. Brown с соавт. [18] изменения размеров отсутствовали в 77% ответивших очагов, а в 23% было отмечено даже их увеличение.
Рис. 4. Форест-график по результатам исследований, определявших изменения значений измеряемого коэффициента диффузии в метастазах в кости, ответивших на лечение.
Интенсивность сигнала на ДВИ. Три исследования [5, 16, 20] содержат данные об изменении интенсивности сигнала в костных метастазах на ДВИ в результате проведённой системной терапии. Во всех работах авторы пришли к однозначному выводу о значительном снижении интенсивности сигнала в ответ на лечение. Однако только S. Cappabianca с соавт. [20] представили числовые значения, указывающие на 35%-ное снижение интенсивности сигнала очагов, ответивших на лучевую терапию.
ИКД. Оценка и анализ количественных значений ИКД проводились в семи работах [5, 9–12, 16, 20]. Н.И. Сергеев с соавт. [16] не приводят данных по каждому пациенту или очагу, но указывают на общее повышение ИКД на 25% в ответ на лечение в остеобластических очагах и снижение на 25% ― в остеолитических. В шести работах [5, 9–12, 20] были представлены точные значения ИКД до и после лечения. Все авторы отмечают увеличение значений ИКД в ответивших на лечение костных метастазах, что отражает форест-график (рис. 4), построенный с помощью модели случайных эффектов, учитывая наличие статистически значимой гетерогенности результатов (p <0,1 для теста χ2 и I2 >40%).
Изменение ИКД в результате лечения в среднем составило +0,35 [+0,12; +0,49] ×10-3 мм2/с. Размах исходных и посттерапевтических значений ИКД по всем шести публикациям представлен на рис. 5 со средними значениями ИКД до лечения и после него 0,83 [0, 71; 1, 03] и 1,18 [0, 83; 1, 49] ×10-3 мм2/с соответственно. Несмотря на частично перекрывающиеся значения, видно, что ИКД у ответивших пациентов в целом был выше, чем до начала лечения.
tDV. В двух исследованиях [11, 12] tDV оценивали по ДВИ с использованием специального программного обеспечения. Средние значения tDV у ответивших пациентов снизились на 59% по данным R. Perez-Lopez с соавт. [11] и на 42,3% по данным M.D. Blackledge с соавт. [12].
Рис. 5. Диаграмма размаха (boxplot) значений измеряемого коэффициента диффузии метастазов в кости до лечения (серый) и через 1–6 мес от начала терапии (сиреневый), построенная с учётом значений всех ответивших очагов (n=156), включённых в публикации.
ОБСУЖДЕНИЕ
Данный систематический обзор демонстрирует возможности использования МРТ для оценки ответа на терапию метастатического поражения костей на основе динамики таких объективных критериев, как размер очагов, относительная интенсивность сигнала на ДВИ, числовые значения ИКД и tDV.
Актуальность рассматриваемого вопроса обусловлена быстро растущим использованием МРТ всего тела для первичной диагностики метастатического поражения костей у больных РПЖ и РМЖ [7, 8, 24, 25], которые в дальнейшем получают различные варианты системной терапии и лучевого лечения и нуждаются в оценке их эффективности.
Оценка размера костных метастазов в качестве объективного показателя эффективности системной терапии используется уже давно. Наиболее часто с этой целью применяют критерии RECIST 1.1 (Response Evaluation Criteria in Solid Tumours), согласно которым к измеряемым поражениям костей относят только остеолитические и смешанные очаги с размером мягкотканного компонента не менее 10 мм. Диффузное поражение костей и остеобластические очаги считаются неизмеримыми [26, 27]. Несмотря на существующие ограничения, критерии RECIST 1.1 для очагов в костях включены в рекомендации MET-RADS-P (METastasis Reporting and Data System for Prostate Cancer) для МРТ всего тела [6] и наряду с ИКД используются для оценки ответа при метастатическом РПЖ [1].
Критерии MDA, разработанные специально для оценки метастазов в кости в Онкологическом центре имени Андерсона Техасского университета (bone-specific response criteria at The University of Texas MD Anderson Cancer Center) [27], позволяют оценивать любые типы очагов на основе динамики их количества, размеров и структурных особенностей. Критерии MDA применимы как для рентгенографии и КТ, так и для МРТ, однако они получили меньшее распространение в сравнении с RECIST 1.1.
Оценка динамики размеров костных метастазов на Т1-ВИ [17–19, 21] показала их сокращение в ответ на специфическую терапию. Только в одном исследовании были получены данные, противоречащие результатам других работ. A.L. Brown с соавт. [18] отметили увеличение ответивших очагов на Т1-ВИ спустя 6–9 мес от начала системного лечения. В этом исследовании все пациенты получали системную или локальную лучевую терапию, из них 17 ― дополнительно химио- или гормонотерапию. Оценка ответа на терапию у этих пациентов проводилась комплексно с использованием клинических, биохимических, рентгенологических и сцинтиграфических критериев. Причиной таких результатов могли быть особенности лечения, приведшие к формированию выраженного, длительно персистирующего перифокального отёка, который даёт гипоинтенсивный сигнал на Т1-ВИ и, согласно практическим рекомендациям Met-RADS, может приводить к ложноположительному увеличению размера ответивших очагов [6]. Таким образом, динамика размеров костных метастазов в ответ на специфическое лечение имеет диагностическую значимость, однако результат может зависеть от времени терапии и отличаться при разных терапевтических подходах и характере метастатических очагов, что указывает на целесообразность дополнения данного критерия другими объективными данными.
Динамика интенсивности сигнала на ДВИ, рассчитанная полуколичественно как отношение интенсивности сигнала от метастатических очагов в костях к интенсивности сигнала от нормальных мышц, может рассматриваться в качестве объективного критерия оценки ответа [5, 16, 20]. Во всех публикациях авторы наблюдали снижение интенсивности сигнала очагов у ответивших на системную терапию пациентов, однако, учитывая, что численные значения представлены только в одной работе [20], а также то, что во всех трёх публикациях не были учтены характер очагов и вид системной терапии, необходимо дальнейшее исследование зависимости изменения интенсивности сигнала на ДВИ от вышеперечисленных условий.
Определение числовых значений ИКД имеет высокий потенциал в качестве объективного оценочного критерия и является одним из ключевых в рекомендациях Met-RADS, предназначенных для анализа МРТ всего тела. Анализ данных семи исследований [5, 9–12, 16, 20] свидетельствует о статистически значимом увеличении ИКД у ответивших пациентов. Вместе с тем во всех работах описаны единичные случаи снижения ИКД при хорошем ответе на лечение. Эти случаи авторы связывают с развитием фиброза или склероза в ответ на терапию. По наблюдениям C. Messiou с соавт. [9], увеличение ИКД может наблюдаться и у пациентов с прогрессией, но менее выраженное, чем у ответивших. Из всех семи публикаций только Н.И. Сергеев и соавт. [16] сопоставляли значения ИКД у ответивших пациентов с характером очага и получили разнонаправленные изменения: повышение ИКД на 25% в остеобластических очагах и снижение на 25% ― в остеолитических. С учётом этих данных отсутствие деления очагов на остеолитические и остеобластические в других исследованиях может быть причиной расхождения в значениях ИКД у ответивших пациентов, а также вносить ограничения в интерпретацию результатов этих работ, что указывает на необходимость дальнейших исследований с включением однородных групп очагов.
В исследованиях, оценивавших общий опухолевый объём (tDV) [11, 12], получены согласующиеся результаты. В обеих публикациях авторы пришли к выводу о значительном снижении tDV в ответ на специфическую терапию. Однако данный подход имеет ряд ограничений. Во-первых, он чувствителен к качеству полученных ДВИ, что может привести к изменению tDV при наличии артефактов или недостаточном подавлении сигнала от окружающих тканей. Во-вторых, в обеих публикациях вручную был удалён сигнал выше 4–5-го шейных позвонков с целью устранения потенциального ложного увеличения tDV от головного мозга, слюнных желёз и большого количества лимфоузлов, что могло привести к пропуску очагов в этих областях. В-третьих, методика времязатратна, т.к. содержит этап ручной обработки, что пока препятствует её широкому использованию в клинической практике.
Таким образом, метод МРТ предлагает комплекс объективных критериев, позволяющих оценивать эффективность терапии диссеминированного метастатического поражения скелета у больных РПЖ и РМЖ, однако для широкого использования его в клинической практике необходимы дополнительные исследования, направленные на уточнение методических аспектов и количественных значений критериев оценки для различных типов метастатических очагов.
Ограничения исследования
Следует указать, что интерпретировать результаты данного систематического обзора следует с осторожностью, учитывая некоторые ограничения, такие как малое количество включённых исследований, ретроспективный характер части работ, отсутствие стандартизированной эталонной методики, разные виды специфической терапии, которые могут влиять на характер микроструктурных изменений и приводить к отличиям МР-характеристик ответивших очагов.
В исследованиях, включённых в систематический обзор и метаанализ, незначительно отличаются параметры сканирования, в частности разное количество и разные значения используемых b-факторов, что может повлиять на итоговые значения ИКД.
ЗАКЛЮЧЕНИЕ
МРТ является информативной методикой для объективной оценки ответа костных метастазов на терапию у больных РПЖ и РМЖ на основе количественных и полуколичественных критериев. В настоящее время при оценке эффективности лечения следует ориентироваться в первую очередь на динамику размеров очагов в режиме Т1 и динамику значений ИКД, причём точные количественные критерии оценки пока не разработаны. Наиболее перспективными являются параметры, базирующиеся на ДВИ, однако это не исключает использования традиционных критериев RECIST 1.1 и MDA.
Таким образом, МРТ всего тела является потенциально эффективным диагностическим инструментом в выявлении и последующем мониторинге метастатического поражения скелета у больных РПЖ и РМЖ.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Авторы заявляют об отсутствии внешнего финансирования при проведении исследования.
Конфликт интересов. Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов. В.О. Рипп ― поиск и анализ литературы, интерпретация литературных данных и написание текста, разработка дизайна работы; Т.П. Березовская ― разработка концепции и дизайна обзора, поиск и анализ литературы, написание текста; С.А. Иванов ― экспертная оценка и итоговая корректировка обзора. Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией).
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. V.O. Ripp ― search and analysis of literature, interpretation of literary data and writing of text, development of work design; T.P. Berezovskaya ― development of the concept and design of the review, search and analysis of literature, text writing; S.A. Ivanov ― expert assessment and final editing of the literature review. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
Об авторах
Владислав Олегович Рипп
Медицинский радиологический научный центр имени А.Ф. Цыба — филиал федерального государственного бюджетного учреждения, «Национальный медицинский исследовательский центр радиологии» Министерства здравоохранения Российской Федерации
Email: rippnba@gmail.com
ORCID iD: 0000-0001-8970-4212
Врач-рентгенолог
Россия, 249036, Обнинск, ул. Королева, д. 4Татьяна Павловна Березовская
Медицинский радиологический научный центр имени А.Ф. Цыба — филиал федерального государственного бюджетного учреждения, «Национальный медицинский исследовательский центр радиологии» Министерства здравоохранения Российской Федерации
Email: berez@mrrc.obninsk.ru
ORCID iD: 0000-0002-3549-4499
главный научный сотрудник отделения МРТ, доктор медицинских наук, профессор
249036, Обнинск, ул. Королева, д. 4Сергей Анатольевич Иванов
Медицинский радиологический научный центр имени А.Ф. Цыба — филиал федерального государственного бюджетного учреждения, «Национальный медицинский исследовательский центр радиологии» Министерства здравоохранения Российской Федерации
Автор, ответственный за переписку.
Email: oncourolog@gmail.com
ORCID iD: 0000-0001-7689-6032
директор МРНЦ им А.Ф. Цыба, доктор медицинских наук, профессор РАН
Россия, 249036, Обнинск, ул. Королева, д. 4Список литературы
- Lecouvet F.E., Larbi A., Pasoglou V., et al. MRI for response assessment in metastatic bone disease//Eur Radiol. 2013. Vol. 23, N 7. Р. 1986–1997. doi: 10.1007/s00330-013-2792-3
- Padhani A.R., Makris A., Gall P., et al. Therapy monitoring of skeletal metastases with whole-body diffusion MRI//J Magn Reson Imaging. 2014. Vol. 39, N 5. Р. 1049–1078. doi: 10.1002/jmri.24548
- Woolf D.K., Padhani A.R., Makris A. Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care?//Ann Oncol. 2015. Vol. 26, N 6. Р. 1048–1057. doi: 10.1093/annonc/mdu558
- Padhani A.R., Gogbashian A. Bony metastases: assessing response to therapy with whole-body diffusion MRI//Cancer Imaging. 2011. Vol. 11, N 1A. Р. S129–S145. doi: 10.1102/1470-7330.2011.9034
- Byun W.M., Shin S.O., Chang Y., et al. Diffusion-weighted MR imaging of metastatic disease of the spine: assessment of response to therapy//AJNR Am J Neuroradiol. 2002. Vol. 23, N 6. Р. 906–912.
- Padhani A.R., Lecouvet F.E., Tunariu N., et al. METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer//Eur Urol. 2017. Vol. 71, N 1. Р. 81–92. doi: 10.1016/j.eururo.2016.05.033
- Zugni F., Ruju F., Pricolo P., et al. The added value of whole-body magnetic resonance imaging in the management of patients with advanced breast cancer//PLoS One. 2018. Vol. 13, N 10. Р. e0205251. doi: 10.1371/journal.pone.0205251
- Yang H.L., Liu T., Wang X.M., et al. Diagnosis of bone metastases: a meta-analysis comparing ¹⁸FDG PET, CT, MRI and bone scintigraphy//Eur Radiol. 2011. Vol. 21, N 12. Р. 2604–2617. doi: 10.1007/s00330-011-2221-4
- Messiou C., Collins D.J., Giles S., et al. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI//Eur Radiol. 2011. Vol. 21, N 10. 2169–2177. doi: 10.1007/s00330-011-2173-8
- Reischauer C., Froehlich J.M., Koh D.M., et al. Bone metastases from prostate cancer: assessing treatment response by using diffusion-weighted imaging and functional diffusion maps ― initial observations//Radiology. 2010. Vol. 257, N 2. Р. 523–531. doi: 10.1148/radiol.10092469
- Perez-Lopez R., Mateo J., Mossop H., et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: a pilot study//Radiology. 2017. Vol. 283, N 1. Р. 168–177. doi: 10.1148/radiol.2016160646
- Blackledge M.D., Collins D.J., Tunariu N., et al. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: a feasibility study//PLoS One. 2014. Vol. 9, N 4. Р. e91779. doi: 10.1371/journal.pone.0091779
- Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration//PLoS Med. 2009. Vol. 6, N 7. Р. e1000100. doi: 10.1371/journal.pmed.1000100
- Amir-Behghadami M., Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews//Emerg Med J. 2020. Vol. 37, N 6. Р. 387. doi: 10.1136/emermed-2020-209567
- Whiting P.F. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies//Ann Intern Med. 2011. Vol. 155, N 8. Р. 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009
- Сергеев Н.И., Котляров П.М., Солодкий В.А. Диффузионно-взвешенная магнитно-резонансная томография в оценке химиолучевого лечения метастатического поражения костных структур//Вестник Российского научного центра рентгенорадиологии Минздрава России. 2016. Т. 16, № 3. С. 2.
- Çiray I., Lindman H., Åström K.G., et al. early response of breast cancer bone metastases to chemotherapy evaluated with mr imaging//Acta Radiologica. 2001. Vol. 42, N 2. Р. 198–206. doi: 10.1080/028418501127346503
- Brown A.L., Middleton G., Macvicar A.D., et al. T1-weighted magnetic resonance imaging in breast cancer vertebral metastases: Changes on treatment and correlation with response to therapy//Clin Radiol. 1998. Vol. 53, N 7. Р. 493–501. doi: 10.1016/s0009-9260(98)80168-2
- Tombal B., Rezazadeh A., Therasse P., et al. Magnetic resonance imaging of the axial skeleton enables objective measurement of tumor response on prostate cancer bone metastases//Prostate. 2005. Vol. 65, N 2. Р. 178–187. doi: 10.1002/pros.20280
- Cappabianca S., Capasso R., Urraro F., et al. Assessing response to radiation therapy treatment of bone metastases: short-term followup of radiation therapy treatment of bone metastases with diffusion-weighted magnetic resonance imaging//J Radiotherapy. 2014. doi: 10.1155/2014/698127
- Котляров П.М., Сергеев Н.И., Федина О.Н. МРТ в диагностике метастатического поражения скелета и в оценке эффективности лечения//Радиология практика. 2006. № 6. С. 10–15.
- Lecouvet F.E., Talbot J.N., Messiou C., et al. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: A review and position statement by the European Organisation for Research and Treatment of Cancer imaging group//Eur J Cancer. 2014. Vol. 50, N 15. Р. 2519–2531. doi: 10.1016/j.ejca.2014.07.002
- Grimm R., Padhani A.R. Whole-body diffusion-weighted MR image analysis with syngo.via frontier MR total tumor//Magn Flash. 2017. Vol. 68, N 2. Р. 73–75.
- Jambor I., Kuisma A., Ramadan S., et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial//Acta Oncol. 2016. Vol. 55, N 1. Р. 59–67. doi: 10.3109/0284186X.2015.1027411
- Heusner T.A., Kuemmel S., Koeninger A., et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging//Eur J Nucl Med Mol Imaging. 2010. Vol. 37, N 6. Р. 1077–1086. doi: 10.1007/s00259-010-1399-z
- Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)//Eur J Cancer. 2009. Vol. 45, N 2. Р. 228–247. doi: 10.1016/j.ejca.2008.10.026
- Costelloe C.M., Chuang H.H., Madewell J.E., et al. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST//J Cancer. 2010. Vol. 1. Р. 80–92. doi: 10.7150/jca.1.80
Дополнительные файлы