Безопасность и эффективность чрескожной сосудопластики с применением устройства Vessel-X при лечении симптоматичных переломов грудных и поясничных позвонков
- Авторы: Masala S.1, Lacchè A.1, Zini C.1, Mannatrizio D.1, Marcia S.1, Bellini M.1, Guglielmi G.1
-
Учреждения:
- University of Foggia
- Выпуск: Том 3, № 2 (2022)
- Страницы: 98-107
- Раздел: Оригинальные исследования
- Статья получена: 18.11.2021
- Статья одобрена: 10.05.2022
- Статья опубликована: 14.07.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/88685
- DOI: https://doi.org/10.17816/DD88685
- ID: 88685
Цитировать
Аннотация
Цель ― оценить результаты клинических и рентгенологических исследований в отношении безопасности и эффективности устройства Vessel-X (Dragon Crown Medical Co., Ltd Shandong, Китай), применяемого для лечения симп-томатичных переломов позвонков с повреждением и без повреждения задней стенки позвонка и/или обеих замыкательных пластинок.
Материалы и методы. Ретроспективно обследовано 66 пациентов, перенёсших 92 хирургических вмешательства в связи с симптоматичными переломами тел позвонков в период с 19 марта по сентябрь 2020 г. Все переломы были разделены на 2 подгруппы: сложные (36 переломов с повреждением задней стенки и/или обеих замыкательных пластинок позвонков) и простые (все остальные). Результаты лечения оценивали по числовой рейтинговой шкале (Numerical rating scale, NRS) и индексу нетрудоспособности Освестри (Oswestry disability index, ODI) за день до хирургического вмешательства и через 1, 6 и 12 мес наблюдения. Восстановление высоты позвонков также оценивали путём сравнения рентгенологических снимков до и после вмешательства.
Результаты. Всего пролечено 92 позвонка (58 поясничных и 34 грудных), в 24 случаях — с помощью многоуровневых процедур. Частота технического успеха составила 100%, выявлен лишь один случай бессимптомной паравертебральной утечки цемента. В обеих подгруппах отмечалась достоверная статистическая разница между показателями NRS и ODI в дооперационный период и через 1, 6 и 12 мес наблюдения (p <0,05), а также в отношении высоты позвонков при сравнении данных до и после операции (p <0,05). Достоверно значимой разницы в отношении восстановления высоты позвонков среди сложных и простых переломов не наблюдалось.
Заключение. Сосудопластика ― безопасный и эффективный метод лечения простых и сложных болезненных переломов позвонков, обеспечивающий значительное уменьшение симптоматики, отличный контроль утечки цемента и надлежащее восстановление высоты позвонков.
Полный текст
ОБОСНОВАНИЕ
В последние десятилетия наблюдается значительное увеличение числа процедур вертебральной аугментации для лечения переломов позвонков, а также развитие более совершенных методик. Все эти вмешательства дают отличные результаты с меньшим количеством осложнений и более высокой рентабельностью в сравнении с традиционными открытыми хирургическими процедурами [1–3].
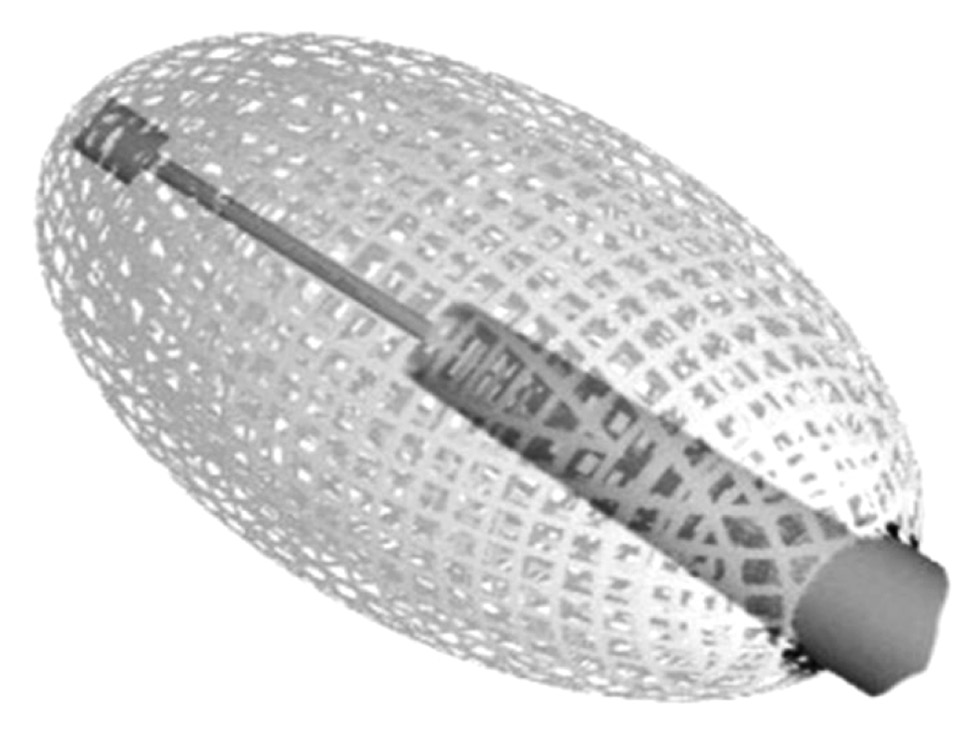
Вертебральная аугментация включает в себя несколько техник лечения: чрескожную вертебропластику, чрескожную кифопластику и методы чрескожной имплантации [4]. Все они направлены на уменьшение и возможное устранение болевых симптомов за счёт консолидации перелома и, по мере возможности, на восстановление парафизиологической высоты тела позвонка путём введения костно-пластического материала (КПМ) под визуальным контролем [4]. Несмотря на то, что введение КПМ при вертебральной аугментации тщательно контролируется с помощью рентгеноскопии, наполнитель может просачиваться за пределы позвонков в соседние пространства в 7–30% случаев [5–7]. В исследовании показана корреляция между введением больших объёмов КПМ и его утечкой. Дополнительный риск возникновения этого осложнения связан с наличием дефектов кортикального слоя кости. Кроме того, чтобы предотвратить нежелательную утечку КПМ в эпидуральное пространство с последующими потенциально серьёзными осложнениями, в нескольких исследованиях были исключены пациенты с дефектами задней стенки позвонка [8]. В последние годы разработано множество устройств для снижения частоты данного осложнения, в том числе Vessel-X (Dragon Crown Medical Co., Ltd Shandong, Китай), используемый в нашем ретроспективном исследовании. Это специальная методика чрескожной имплантации с использованием контейнера из полиэтилентерефталата (нерастяжимый материал с пористостью 100 мкм) для восстановления высоты тела позвонка и предотвращения утечки КПМ. Более того, когда давление внутри контейнера превышает окружающее сопротивление, КПМ начинает интердигитировать через поры равномерно во всех направлениях, не скапливаясь в области малого сопротивления тела позвонка (рис. 1).
Рис. 1. Устройство Vessel-X, изготовленное из сетчатого нерастяжимого материала (полиэтилентерефталат) пористостью 100 мкм.
МАТЕРИАЛЫ И МЕТОДЫ
Дизайн исследования
Ретроспективное одноцентровое исследование на относительно небольшой выборке.
Критерии соответствия
Критерии включения: наличие болезненных переломов тел позвонков в поясничном отделе, резистентных к консервативному лечению.
Критерии исключения: наличие активной инфекции, перелома или аномального тела позвонка без болей или клинических проблем, старых переломов, коагулопатии; ущемление спинного мозга или нерва, вызывающее радикулярные боли, с остеобластическими или костными метастазами, распространяющимися в эпидуральное пространство; другие процедуры вертебральной аугментации в анамнезе.
Описание медицинского вмешательства
Оценка по цифровой рейтинговой шкале (Numerical rating scale, NRS) и индексу инвалидности Освестри (Oswestry disability index, ODI) проводилась за 1 день до процедуры и через 1, 6, 12 мес после неё. Обследование с помощью магнитно-резонансной томографии (МРТ) проводили через 1, 6 и 12 мес, с помощью компьютерной томографии (КТ) ― через 1 и 12 мес после вмешательства.
Высоту тела позвонка в предоперационный период определяли путём измерения высоты в заднем, центральном и переднем отделе тела позвонка с помощью МРТ и/или КТ, в послеоперационный ― сразу после имплантации устройства Vessel-X по результатам рентгеноскопии через 1, 6, 12 мес (МРТ) и через 1 и 12 мес (КТ).
Восстановление высоты позвонков рассчитывали путём измерения разницы между высотой до и после операции с контрольным измерением соседних нелеченых тел позвонков в качестве референсного значения.
Процедуры проводились в специализированном отделении сосудистой хирургии под местной анестезией и постоянной антибиотикопрофилактикой в соответствии с рекомендациями Европейского общества кардиоваскулярной и инвазивной радиологии [4].
Пациента укладывали в положение лёжа, под контролем рентгеноскопии центрировали целевой позвонок и поворачивали дугу для отображения выбранного транспедикулярного пути. Затем вводили троакар (спинальная игла диаметром 8G с переменной длиной 90–150 мм) до достижения тела позвонка. Целевая точка ― область за задней стенкой позвонка в боковой проекции и по направлению к средней линии в переднезадней проекции между двумя замыкательными пластинками.
Vessel-X представлен в двух размерах. В предоперационный период предпочтение отдавалось тому варианту, который соответствовал размеру позвонка. После имплантации устройства вводили КПМ (рентгеноконтрастный костный цемент высокой вязкости на акриловой основе) в объёме 3,3±0,8 мл на позвонок.
После заполнения костным материалом Vessel-X принимал цилиндрическую форму согласно заданным размерам. Максимальное давление КПМ внутри контейнера, прежде чем наполнитель начинает выходить наружу, связано с относительным сопротивлением плотности окружающей кости, различной при новых («свежих») и старых переломах, а также в случае с молодой костной тканью или тканью с остеопоротическими изменениями. По достижении этого давления наполнитель проникал в микропоры, интердигитируя в трабекулярное пространство, и стабилизировал контейнер с последующим поднятием замыкательных пластинок позвонков. Техническим успехом считалось корректное размещение и успешная имплантация устройства (рис. 2, 3).
Рис. 2. Размещение устройства Vessel-X: а–d ― интраоперационная имплантация (костно-пластический материал начинает выходить из ПЭТ-контейнера только по достижении максимального размера; e ― объёмное преобразование устройства.
Рис. 3. Идеальное размещение устройства Vessel-X без утечки костно-пластического материала: а ― сагиттальная реконструкция сложного перелома позвонка на компьютерной томографии; b ― послеоперационный компьютерно-томографический контроль.
Этическая экспертиза
Официальное разрешение для данного вида исследования не требуется.
Статистический анализ
Показатели NRS и ODI были представлены в виде описательных статистических данных (среднее значение, стандартное отклонение, медиана, интерквартильный размах), оцениваемых до операции и через 1, 6, 12 мес наблюдения.
Для выявления статистически значимых изменений в показателях NRS и ODI в период после лечения и сравнения результатов с периодом, предшествующим лечению, использовали парный t-критерий Стьюдента и знаковый ранговый критерий Вилкоксона. Для каждой серии оценок принимали нулевую гипотезу об отсутствии различий между результатами, полученными до и после лечения.
Все виды анализа выполняли с помощью пакета прикладных программ Matlab (The MathWorks Inc., Натик, Массачусетс, США).
РЕЗУЛЬТАТЫ
Объекты (участники) исследования
В исследование включены 66 пациентов (16 мужчин и 50 женщин, средний возраст 73,1 года) с болезненными переломами тел позвонков в поясничном отделе, резистентными к консервативному лечению. Пациенты с переломом замыкательной пластинки и/или дефектом задней стенки позвонка, выявленными с помощью МРТ и/или КТ, вошли в подгруппу сложных переломов, связанных с остеопорозом (n=19), травмой (n=3), множественной миеломой (n=2), метастатическим раком молочной железы (n=2).
Всего пролечено 92 позвонка (58 поясничных и 34 грудных, диапазон D5–L5) с использованием устройства Vessel-X. Многоуровневая процедура в рамках одного и того же вмешательства проведена 24 пациентам (3 уровня ― 2 пациентам; 2 уровня ― 22 пациентам). На поясничном уровне применяли бипедикулярный доступ, на грудном ― монопедикулярный. Технический успех составил 100%.
Основные результаты исследования
Серьёзных осложнений не наблюдалось. При контрольных МРТ и КТ через 1 мес после операции выявлен единичный случай бессимптомной паравертебральной утечки наполнителя у пациента с травматическим переломом тела позвонка L1 без повреждения спинномозгового канала и нервных корешков. В течение 12-месячного периода наблюдения новых переломов тел соседних позвонков не выявлено.
У 10 пациентов с патологическими и травматическими переломами МРТ через 6 и 12 мес после вмешательства подтвердила отсутствие отёка костного мозга в целевом и соседних позвонках.
Мы наблюдали значительное снижение предоперационных средних значений ODI (73,2±7,9) через 1 мес (14,1±3,3), 6 мес (13,8±3,6) и 12 мес (14,0±2,9) наблюдения; p <0,05 (рис. 4).
Рис. 4. Индекс выражается в процентах и варьирует от 0 до 100%, где нижний предел означает отсутствие инвалидности, верхний ― максимальную степень инвалидности (пациенты прикованы к постели). До лечения средний показатель ODI составлял 78% (25-й процентиль ― 70,5%, 75-й процентиль ― 84%). Выпадающие значения не выявлены. Через 1 мес после лечения средний показатель ODI составлял 14% (25-й процентиль ― 12,7%, 75-й процентиль ― 17%). Выпадающие значения не выявлены. Через 6 мес после лечения средний показатель ODI составлял 13% (25-й процентиль ― 12%, 75-й процентиль ― 16%). Выпадающие значения не выявлены. Через 12 мес после лечения средний показатель ODI составлял 13% (25-й процентиль ― 12,4%, 75-й процентиль ― 16%). Выпадающие значения не выявлены. Средний балл по шкале ODI снизился с 73,2±7,9 до 14,1±3,3 через 1 мес и до 13,8±3,6 через 6 мес после операции (p <0,001).
Предоперационные средние значения NRS (7,3±1,2) также снизились через 1 мес (1,8±1,3), 6 мес (2,1±0,8) и 12 мес (1,7±1,0) после операции; p <0,05 (рис. 5).
Рис. 5. До лечения показатели NRS в основном были сосредоточены на верхних границах шкалы: медиана 8, 25-й процентиль 7, 75-й процентиль 8. Распределение баллов NRS после лечения по результатам опроса: через 1 мес ― медиана 2, 25-й процентиль 2, 75-й процентиль 3; через 6 мес ― медиана 2, 25-й процентиль 2, 75-й процентиль 3; через 12 мес ― медиана 2; 25-й процентиль 2; 75-й процентиль 3. Выпадающие значения не выявлены. До лечения средний показатель NRS составлял 7,3±1,2 и снизился до 1,8±1,3 через 1 мес, до 2,1±0,8 через 6 мес и 1,7±1,0 через 12 мес после операции (p <0,001).
Статистически достоверных различий в двух подгруппах переломов позвонков по стандартному отклонению не наблюдалось.
Средние значения высоты тела позвонка переднего отдела в предоперационный период увеличились после процедуры с 11,3±2,2 мм (диапазон 7–15) до 14,0±1,7 мм (диапазон 10–19) (p <0,05), тогда как центрального и заднего отделов ― с 11,9±2,5 мм (диапазон 6–17) до 16,1±1,8 мм (p <0,05) и с 16,4±2,5 мм (диапазон 10–22) до 19,5±1,6 мм (диапазон 23–16) в аналогичный период соответственно (p <0,05).
Статистически достоверных различий по показателям высоты тела позвонка между подгруппами с простыми и сложными переломами не наблюдалось.
При переломах позвонков, пролеченных с использованием двустороннего доступа, распределение КПМ было более однородным, чем при монопедикулярном доступе, однако в отношении высоты тела позвонка различий между бипедикулярной и монопедикулярной группами не выявлено.
ОБСУЖДЕНИЕ
К одному из основных осложнений вертебральной аугментации относится нежелательная утечка цемента за пределы тела позвонка. Масштабный метаанализ, проведённый Y. Zhan и соавт. [9], показал, что частота утечки цемента при чрескожной вертебропластике составляет 54,7%, при чрескожной баллонной кифопластике ― 18,4%.
Для снижения риска утечки цемента было разработано множество устройств, включая Vessel-X. Результаты краткосрочного наблюдения в нашем исследовании ― многообещающие. Полный технический успех с единственным случаем бессимптомной утечки цемента (1,08%) свидетельствует о том, что сосудопластика является безопасным и эффективным методом лечения переломов позвонков, в том числе при переломе замыкательных пластинок и/или задней стенки позвонка с высоким риском нежелательных явлений. Клинически значимых побочных эффектов, инфекций или повреждений нервной ткани не выявлено.
Кроме того, показано, что вытекание костного цемента в межпозвонковое пространство повышает риск новых переломов соседних позвонков [10–16]. В нашем исследовании утечки цемента в межпозвонковое пространство не наблюдалось, и в течение 12 мес после операции ни одного нового перелома не выявлено. Полагаем, что это связано со свойствами системы Vessel-X, которая обеспечивает контролируемое распределение КПМ за счёт его однородного распределения через поры сетчатого материала в сравнении с другими устройствами чрескожной имплантации, где цемент скапливается в первую очередь по линии перелома, в результате чего происходит его утечка [17, 18].
Помимо профилактики осложнений, сосудопластика показала отличные клинические результаты, подтверждённые значительным снижением показателей NRS и ODI во время последующих оценок.
Сообщается, что введение КПМ с использованием бипедикулярного доступа даёт лучшие результаты в восстановлении жёсткости тел позвонков, хотя существенных отличий в прочности костной ткани не отмечалось, что объясняется большим объёмом вводимого КПМ и его симметричным распределением [18]. На грудном уровне, там, где это было возможно, применялся монопедикулярный доступ, для поясничного отдела позвоночника использовался бипедикулярный доступ, в основном из-за более высокого осевого давления, что, по нашему мнению, требовало большее количество КПМ. Что касается восстановления высоты позвонков, существенной разницы между этими двумя подходами не наблюдалось.
Если говорить о восстановлении высоты позвонков, в послеоперационный период необходимо обеспечить стабильность перелома. Несколько исследований показали, что после чрескожной кифопластики высота тела позвонка регулярно снижается, вероятно, по причине неоднородного распределения КПМ. При чрескожной кифопластике баллон внутри тела позвонка раздувается, а затем извлекается для последующего введения цемента, что вызывает частичный коллапс тела позвонка и негативно сказывается на восстановлении его высоты [5, 17, 18].
При стандартной чрескожной вертебропластике и нестабильных переломах это событие встречается чаще. В таких случаях рекомендуется ещё одна процедура чрескожной вертебропластики, однако следует отметить, что риск нежелательной утечки цемента при этом значительно возрастает [9, 19–22].
В нашем исследовании не наблюдалось ощутимой разницы в показателях высоты тела позвонка ни сразу после процедуры, ни в конце периода наблюдения, что является подтверждением того, что устройство Vessel-X обеспечивает хорошую поддержку сломанному позвонку и предотвращает его коллапс. Кроме того, сосудопластика сокращает время воздействия ионизирующего излучения, поскольку при введении первых 2 мл КПМ рентгеноскопический контроль не требуется. Каждые последующие 0,25 мл КПМ вводятся под контролем рентгеноскопии до достижения желаемой высоты позвонка [17].
Ограничения исследования
Ограничения исследования связаны с его одноцентровым ретроспективным характером и относительно небольшим размером выборки. Однако мы получили обнадёживающие результаты, что в случае их подтверждения позволит лечить переломы задней стенки позвонков.
ЗАКЛЮЧЕНИЕ
Сосудопластика с использованием устройства Vessel-X ― эффективный и безопасный метод лечения стандартных и сложных переломов позвонков.
Благодаря особой конструкции, Vessel-X обеспечивает оптимальный контроль распределения КПМ, уменьшение частоты утечки цемента и применения рентгеноскопии по сравнению с чрескожной вертебро- и кифопластикой.
Vessel-X показал хорошие клинические результаты со значительным снижением показателей числовой рейтинговой шкалы (NRS) и индекса нетрудоспособности (ODI) после лечения, однако для дальнейшего подтверждения полученных результатов необходимо проводить проспективные рандомизированные исследования.
ДОПОЛНИТЕЛЬНО
Источник финансирования. Авторы заявляют об отсутствии внешнего финансирования при проведении исследования.
Конфликт интересов. Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Вклад авторов. Все авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией). Наибольший вклад распределён следующий образом: S. Masala ― концепция исследования, его проведение и редактирование рукописи; A. Lacchè ― сбор, анализ, интерпретация данных исследования; Ch. Zini ― анализ и интерпретация данных для исследования; D. Mannatrizio ― написание текста рукописи и его редактирование; S. Marcia ― интерпретация данных исследования; M. Bellini, G. Guglielmi ― написание текста рукописи и его редактирование.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. S. Masala ― conception of the work, drafting and revising the work; A. Lacchè ― acquisition, analysis, interpretation of data for the work; Ch. Zini ― analysis, interpretation of data for the work; D. Mannatrizio ― drafting and revising the work; S. Marcia ― interpretation of data for the work; M. Bellini, G. Guglielmi ― drafting and revising the work.
Об авторах
Salvatore Masala
University of Foggia
Email: salva.masala@tiscali.it
ORCID iD: 0000-0003-0032-7970
MD
Италия, ФоджаAdriano Lacchè
University of Foggia
Email: adrianolacche@gmail.com
ORCID iD: 0000-0003-1782-8624
MD
Италия, ФоджаChiara Zini
University of Foggia
Email: zini.chiara@gmail.com
ORCID iD: 0000-0003-3456-4106
MD
Италия, ФоджаDomenico Mannatrizio
University of Foggia
Email: dr.mannatrizio@gmail.com
ORCID iD: 0000-0003-3365-7132
MD
Италия, ФоджаStefano Marcia
University of Foggia
Email: stemarcia@gmail.com
ORCID iD: 0000-0002-2118-9864
MD
Италия, ФоджаMatteo Bellini
University of Foggia
Email: matteo.bellini@icloud.com
ORCID iD: 0000-0002-1704-6246
MD
Италия, ФоджаGiuseppe Guglielmi
University of Foggia
Автор, ответственный за переписку.
Email: giuseppe.guglielmi@unifg.it
ORCID iD: 0000-0002-4325-8330
MD, Professor
Италия, ФоджаСписок литературы
- Kushchayev S.V., Wiener P.C., Teytelboym O.M., et al. Percutaneous vertebroplasty: a history of procedure, technology, culture, specialty, and economics // Neuroimaging Clin N Am. 2019. Vol. 29, N 4. Р. 481–494. doi: 10.1016/j.nic.2019.07.011
- Bornemann R., Koch E.M., Wollny M., Pflugmacher R. Treatment options for vertebral fractures an overview of different philosophies and techniques for vertebral augmentation // Eur J Orthop Surg Traumatol. 2014. Vol. 24, Suppl 1. Р. S131–143. doi: 10.1007/s00590-013-1257-3
- Flors L., Lonjedo E., Leiva-Salinas C., et al. Vesselplasty: a new technical approach to treat symptomatic vertebral compression fractures // AJR Am J Roentgenol. 2009. Vol. 193, N 1. Р. 218–226. doi: 10.2214/AJR.08.1503
- Tsoumakidou G., Too C.W., Koch G., et al. CIRSE guidelines on percutaneous vertebral augmentation // Cardiovasc Intervent Radiol. 2017. Vol. 40, N 3. Р. 331–342. doi: 10.1007/s00270-017-1574-8
- Filippiadis D.K., Marcia S., Masala S., et al. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies // Cardiovasc Intervent Radiol. 2017. Vol. 40, N 12. Р. 1815–1823. doi: 10.1007/s00270-017-1779-x
- Diel P., Röder C., Perler G., et al. Radiographic and safety details of vertebral body stenting: results from a multicenter chart review // BMC Musculoskelet Disord. 2013. Vol. 14. Р. 233. doi: 10.1186/1471-2474-14-233
- Vanni D., Galzio R., Kazakova A., et al. Third-generation percutaneous vertebral augmentation systems // J Spine Surg. 2016. Vol. 2, N 1. Р. 13–20. doi: 10.21037/jss.2016.02.01
- Anselmetti G.C., Manca A., Marcia S., et al. Vertebral augmentation with nitinol endoprosthesis: clinical experience in 40 patients with 1-year follow-up // Cardiovasc Intervent Radiol. 2014. Vol. 37, N 1. Р. 193–202. doi: 10.1007/s00270-013-0623-1
- Zhan Y., Jiang J., Liao H., et al. Risk factors for cement leakage after vertebroplasty or kyphoplasty: a meta-analysis of published evidence // World Neurosurg. 2017. Vol. 101. Р. 633–642. doi: 10.1016/j.wneu.2017.01.124
- Tempesta V., Cannata G., Ferraro G., et al. The new vessel-x kyphoplasty for vertebral compression fractures: 2-year follow-up of 136 levels. Las Vegas: American Academy of Orthopaedic Surgeons Annual Meeting; 2009.
- McCall T., Cole C., Dailey A. Vertebroplasty and kyphoplasty: a comparative review of efficacy and adverse events // Curr Rev Musculoskelet Med. 2008. Vol. 1. Р. 17–23. doi: 10.1007/s12178-007-9013-0
- Mroz T.E., Yamashita T., Davros W.J., Lieberman I.H. Radiation exposure to the surgeon and the patient during kyphoplasty // J Spinal Disord Tech. 2008. Vol. 21, N 2. Р. 96–100. doi: 10.1097/BSD.0b013e31805fe9e1
- Ruiz S.F., Santiago C.A., Guzmán Á.L., et al. Comparative review of vertebroplasty and kyphoplasty // World J Radiol. 2014. Vol. 6, N 6. Р. 329–343. doi: 10.4329/wjr.v6.i6.329
- Hiwatashi A., Yoshiura T., Yamashita K., et al. Morphologic change in vertebral body after percutaneous vertebroplasty: follow-up with MDCT // AJR Am J Roentgenol. 2010. Vol. 195. Р. W207–W212. doi: 10.2214/AJR.10.4195
- Grohs J.G., Matzner M., Trieb K., Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty // J Spinal Disord Tech. 2005. Vol. 18, N 3. Р. 238–242.
- Lin E.P., Ekholm S., Hiwatashi A., Westesson P.L. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body // AJNR Am J Neuroradiol. 2004. Vol. 25, N 2. Р. 175–180.
- Bambang D. Vesselplasty: a novel concept of percutaneous treatment for stabilization and height restoration of vertebral compression fractures // J Musculoskeletal Res. 2008. Vol. 11, N 2. Р. 71–79. doi: 10.1142/s0218957708001985
- Zheng Z., Luk K.D., Kuang G., et al. Vertebral augmentation with a novel Vessel-X bone void filling container system and bioactive bone cement // Spine (Phila Pa 1976). 2007. Vol. 32, N 19. Р. 2076–2082. doi: 10.1097/BRS.0b013e3181453f64
- Carlier R.Y., Gordji H., Mompoint D.M., et al. Osteoporotic vertebral collapse: percutaneous vertebroplasty and local kyphosis correction // Radiology. 2004. Vol. 233, N 3. Р. 891–898. doi: 10.1148/radiol.2333030400
- Chen W.J., Kao Y.H., Yang S.C., et al. Impact of cement leakage into disks on the development of adjacent vertebral compression fractures // J Spinal Disord Tech. 2010. Vol. 23, N 1. Р. 35–39. doi: 10.1097/BSD.0b013e3181981843
- Komemushi A., Tanigawa N., Kariya S., et al. Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture // Cardiovasc Intervent Radiol. 2006. Vol. 29, N 4. Р. 580–585. doi: 10.1007/s00270-005-0138-5
- Guarnieri G., Masala S., Muto M. Update of vertebral cementoplasty in porotic patients // Interv Neuroradiol. 2015. Vol. 21, N 3. Р. 372–380. doi: 10.1177/1591019915582364
Дополнительные файлы