Safety and efficacy of percutaneous vesselplasty (Vessel-X) in the treatment of symptomatic thoracolumbar vertebral fractures
- Authors: Masala S.1, Lacchè A.1, Zini C.1, Mannatrizio D.1, Marcia S.1, Bellini M.1, Guglielmi G.1
-
Affiliations:
- University of Foggia
- Issue: Vol 3, No 2 (2022)
- Pages: 98-107
- Section: Original Study Articles
- Submitted: 18.11.2021
- Accepted: 10.05.2022
- Published: 14.07.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/88685
- DOI: https://doi.org/10.17816/DD88685
- ID: 88685
Cite item
Abstract
AIMS: to assess radiological and clinical outcomes, in terms of safety and efficacy, of symptomatic vertebral fractures with and without posterior wall and\or both endplates involvement, treated with vesselplasty technique (Vessel-X, Dragon Crown Medical Co., Ltd Shandong, China).
MATERIALS AND METHODS: We retrospectively evaluated 66 Patients who underwent 92 vesselplasty procedures, performed for the treatment of symptomatic vertebral body fractures from March 19 to September 2020. We divided the fractures in two subgroups: 36 vertebral fractures with posterior wall and/or both endplates involvement, which we defined complex, while all the others were defined simple. Numerical Rating Scale (NRS) and Oswestry Disability Index (ODI) values has been registered 1 day before the procedure and at 1, 6 and 12 months follow-up. We also evaluated vertebral height restoration by comparing pre-interventional with post-interventional imaging.
RESULTS: 92 vertebrae were treated (58 lumbar, 34 thoracic), with 24 multilevel procedures. We observed a technical success rate of 100%, without major complications; a single case of asymptomatic paravertebral cement leak was reported. Both simple and complex subgroups registered a significative statistical difference in NRS and ODI between preoperative and at 1, 6 and 12 months (p <0.05). A significant statistical difference was demonstrated in vertebral height comparing pre-operative and post-operative data (p <0.05). No significant difference in vertebral height restoration was observed between simple and complex vertebral fractures groups.
CONCLUSIONS: Vesselplasty represents a safe and effective technique for the treatment of both simple and complex painful vertebral fractures, granting a significant reduction of symptoms, excellent cement leakage control and proper vertebral height restoration.
Full Text
INTRODUCTION
In the last decades, a significant increase has been observed in the number of vertebral augmentation procedures (VAP) for the treatment of vertebral fractures (VFs), with the development of more advanced techniques. All these interventions cause excellent results, with reduced complication rates and a better cost–benefit ratio compared to open surgical procedures [1-3].
The VAP term comprises several treatment options that can be subdivided into simple percutaneous vertebroplasty (PVP), percutaneous kyphoplasty (PKP), and percutaneous implant techniques (PIT) [4], which all aim to reduce and possibly eliminate pain symptoms by fracture consolidation and, whenever is possible, to restore paraphysiological vertebral body height (VBH) by injecting bone-filling material (BFM) under image guidance [4].
Although BFM injection is thoroughly monitored via fluoroscopy imaging, it can leak outside the vertebrae into the adjacent spaces in 7%–30% of VAPs [5-7].
A correlation between high volume injections of BFM and its leakage has been demonstrated; the presence of cortical bone defects of the vertebra represents an additional risk factor for the occurrence of this complication. Moreover, to prevent an undesired BFM leakage in the epidural space with potentially serious complications, several studies excluded patients with posterior wall defects [8].
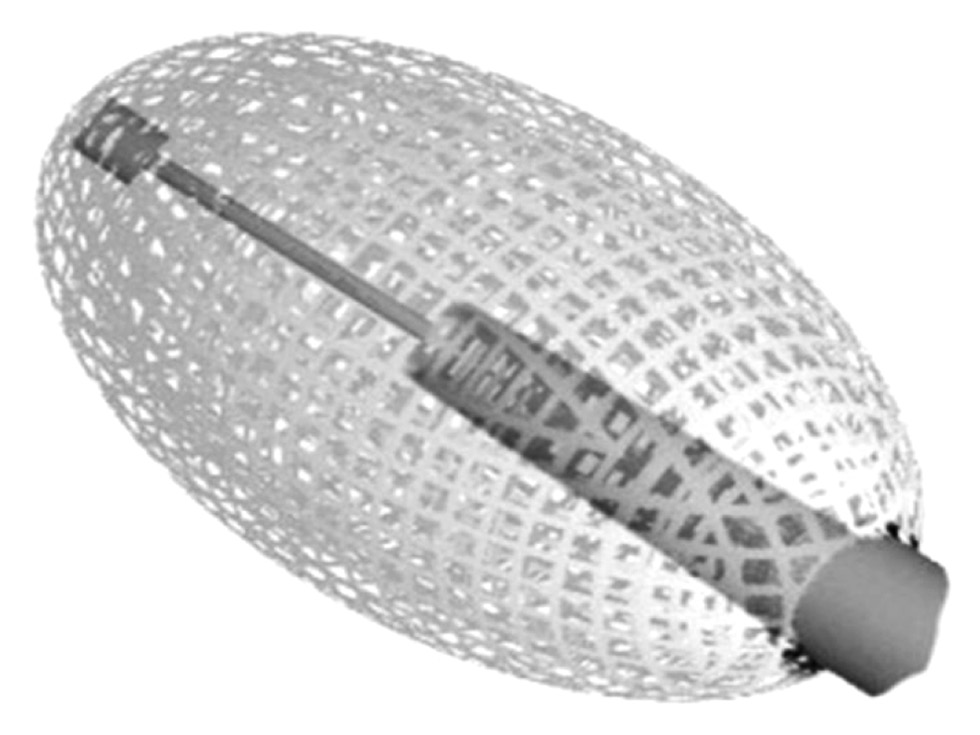
Many devices have been developed during the last years to reduce the incidence of this complication; therefore, our retrospective study aimed to share our experiences in using the vesselplasty technique (Vessel-X, Dragon Crown Medical Co., Ltd Shandong, China), a dedicated PIT with a container made of polyethylene terephthalate (PET), a non-stretchable material with 100-μm porosity, to restore the VBH and prevent BFM leakage. Furthermore, when the pressure inside the container is greater than the surrounding resistance, BFM starts to interdigitate through the pores equally in all directions, without concentrating in the locus of minor resistance of the vertebral body (Fig. 1).
Fig. 1. Vessel-X device, made of a mesh of Polyethylene Terephthalate (PET), a non-stretchable material that has a porosity of 100-μm.
METHODS AND MATERIALS
Study design
A retrospective single-center study on a relatively small sample size.
Patients
In our study, 66 patients (16 males and 50 females, with a mean age of 73.1 year) with 92 painful dorsolumbar vertebral body fractures who were resistant to conservative management were included in the study; among them, 56 were associated with severe osteoporosis, 4 with multiple myeloma, 2 with metastatic breast cancer, and 4 were traumatic.
Patients with active infection, a fracture, or an abnormal vertebral body that is not causing pain or clinical problem; with very old fractures, coagulopathy, spinal cord or nerve impingement causing radicular pain, osteoblastic metastasis, and bone metastasis that extended to the epidural space; and who already underwent other VAP were excluded.
Patients with endplate interruption and/or posterior wall defect documented with magnetic resonance (MR) and/or computed tomography (CT) were classified to the “complex” subgroup, which had VFs related to osteoporosis (N = 19), traumatic fracture (N = 3), multiple myeloma (N = 2), and metastatic breast cancer (N = 2).
A total of 24 patients underwent a multilevel procedure in the same intervention (3 levels, N = 2; 2 levels, N = 22).
The numerical rating scale (NRS) score and Oswestry disability index (ODI) have been evaluated 1 day before the procedure and at 1, 6, and 12 months after.
MRI follow-up was performed at 1, 6, and 12 months, whereas CT was performed at 1 and 12 months post-intervention.
Imaging analysis
Preoperative VBH was obtained by measuring anterior, central, and posterior heights with MRI and/or CT; postoperative VBH was calculated immediately after the Vessel-X implantation with fluoroscopic images and at 1, 6, and 12 months post-intervention with MRI and at 1 and 12 months with CT.
Vertebral height restoration (VHR) was calculated by measuring the difference between pre- and postoperative height, with control measurement of the adjacent untreated vertebral bodies as a reference.
Procedure
Procedures have been performed in our dedicated angiosuite under local anesthesia and ongoing antibiotic prophylaxis based on CIRSE guidelines [4].
The patient was placed in the prone position, and, under fluoroscopic guidance, the target vertebra was centered and the arc rotated to display the chosen transpedicular path. Then, the trocar, a spinal needle of 8-G with a variable length of 90–150 mm was advanced until it reached the vertebral body.
The target position lies immediately after the posterior wall in lateral projection and toward the midline in A–P projection and at midway between the two endplates.
Vessel-X has two dimensions and we chose which one to use in the preoperative based on the vertebral size. Then, it was positioned and filled with BFM (mean quantity = 3.3±0.8 mL/vertebra). BFM is a high viscosity acrylic-based radiopaque bone cement.
After injecting BFM, Vessel-X loses its tubular shape in favor of a cylindrical conformation until a predefined size is reached.
The BFM maximum pressure inside the container, before it starts to spread outside, is associated with the relative resistance of the surrounding bone density, which is different between fresh and old fractures or young and osteoporotic bone. Once this pressure is reached, it starts to penetrate the micropores interdigitating between the trabecular spaces and stabilizing the container with the subsequent lifting of vertebral endplates.
Technical success was defined as the correct placement and implant of the Vessel-X (Fig. 2).
Fig. 2. (a–d): Intraoperatory positioning of Vessel-X device. BMF starts to spread out of the PET container only after it reached its maximum size. (e): VR reconstruction of Vessel-X.
Ethical statement
Formal consent is not required for this type of study.
Statistical analysis
NRS and ODI were presented as descriptive statistics, such as the mean, standard deviation, median, and interquartile range, before and at 1-, 6-, and 12-months follow-up.
To detect statistically significant changes in NRS and ODI in the post-treatment period and to compare the results with the pre-treatment period, both paired t-test and Wilcoxon matched-paired signed-rank test were used.
The null hypothesis of no difference between pre- and post-treatment was then assumed for each series of scores.
All analyses were performed using Matlab (The MathWorks Inc., Natick, MA, USA).
RESULTS
A total of 92 vertebrae have been treated (58 lumbar and 34 thoracic; range, D5–L5) using Vessel-X with a technical success rate of 100%.
The bipedicular approach was the preferred method at lumbar levels, whereas the monopedicular approach was performed in the thoracic vertebrae.
A multilevel procedure was performed in 24 patients (3 levels in 2; 2 levels in 22).
No major complication occurred; a single case of asymptomatic paravertebral leak in a L1 traumatic complex fracture without involving the spinal canal or nerve roots was observed in both MRI and CT control at 1 month.
No new fractures in the adjacent vertebral bodies were reported during the 12-month follow-up period.
In 10 patients with pathological and traumatic fractures, MRI scan at 6 and 12 months confirmed the absence of bone marrow edema in the target vertebra and the adjacent ones.
We observed a significant decrease in ODI values from a preoperative mean of 73.2±7.9 to the mean values of 14.1±3.3, 13.8±3.6, and 14.0±2.9 at 1-, 6-, and 12-months follow-up, respectively (p < 0.05) (Fig. 3).
Fig. 3. (a): CT scan: Sagittal reconstruction of a complex vertebral fracture. (b): Post-operative CT control. Vessel-X is perfectly placed without BFM leakage.
The preoperative mean NRS of 7.3±1.2 dropped to 1.8±1.3, 2.1±0.8, and 1.7±1.0 at 1, 6, and 12 months (p < 0.05). (Fig. 4)
Fig. 4. The index is expressed in percentage points and ranges from 0% to 100%, with the lower limit related to the absence of disability and the upper limit to the maximum degree of disability (patients are bed-bound). At pretreatment, the median ODI score was 78% (25th percentile, 70.5%; 75th percentile, 84%); no outliers were identified. At 1 month post-treatment, the median ODI score was 14% (25th percentile, 12.7%; 75th percentile, 17%); no outliers were identified. At 6 months post-procedure, the median ODI score was 13% (25th percentile, 12%; 75th percentile, 16%); no outliers were identified. At 12 months post-procedure, the median ODI score was 13% (25th percentile, 12.4%; 75th percentile, 16%); no outliers were identified. The mean ODI scores decreased from 73.2±7.9 to 14.1±3.3 at 1 month and 13.8±3.6 at 6 months (p < 0.001).
Fig. 5. At pretreatment, NRS scores were mostly concentrated on the upper limits of the scale (median, 8; 25th percentile, 7; and 75th percentile, 8). The distribution of NRS scores in the post-treatment survey at 1 month (median, 2; 25th percentile, 2; and 75th percentile, 3) at 6 months (median, 2; 25th percentile, 2; and 75th percentile, 3), and 12 months (median, 2; 25th percentile, 2; and 75th percentile, 3); no outlier was identified. The mean NRS score was 7.3±1.2 at pre-procedure and decreased to 1.8±1.3 at 1 month, 2.1±0.8 at 6 months, and 1.7±1.0 at 12 months (p < 0.001).
No statistically significant difference was observed in the two VF subgroups based on standard deviation.
The mean preoperative anterior VBH was 11.3±2.2 (range 7–15) mm and increased to 14.0±1.7 (range 10–19) mm) at post-procedure (p < 0.05).
The mean preoperative central VBH was 11.9±2.5 (range 6–17) mm and increased to 16.1±1.8 mm at post-procedure (p < 0.05).
Mean preoperative posterior VBH was 16.4±2.5 (range 10–22) mm and increased to 19.5±1.6 (range 23–16) mm at post-procedure (p < 0.05).
No statistically significant differences were observed based on VHR between the simple and complex VF subgroups.
In VBs treated with bilateral access, the distribution of BFM was more homogeneous than in the monopedicular approach. However, no difference was observed in VBH restoration between bipedicular and monopedicular groups.
DISCUSSION
One of the main complications of VAP is represented by undesired cement leakage outside the target vertebral body. A large meta-analysis conducted by Zhan Y. et al. showed an incidence rate of cement leakage of 54.7% and 18.4% for percutaneous vertebroplasty and percutaneous balloon kyphoplasty, respectively [9].
To reduce the risk of cement leakage, many devices have been developed including the Vessel-X. In our study, short-term follow-up results are promising; the complete technical success with just a single case of asymptomatic cement leakage (1.08%) indicates that vesselplasty is a safe and effective technique for VF treatment, including those with endplates and/or posterior wall interruption highly at risk for adverse events. No clinically significant side effects, infection, or neural damages were observed.
Additionally, intradiscal cement leak has been shown to increase the risk of subsequent new fractures of the adjacent vertebral bodies [10–16]; in our study, no intradiscal cement leak occurred and no subsequent fracture has been demonstrated at the 12-month follow-up. We believe this is related to Vessel-X properties of a controlled BFM distribution due to a homogeneous spread through its mesh pores, in contrast to other PIT devices in which the cement expansion privileges the weakest areas of the fracture, causing leakage [17, 18].
Beyond complication prevention, vesselplasty has shown excellent clinical results, supported by the significant reduction in NRS and ODI values during follow-up evaluations.
Bipedicular injection of BFM is reported to provide better results based on VB stiffness restoration, albeit no significant difference in VB strength, due to the greater volume of BFM applied and the symmetric distribution [18]. When possible, we opted for a monopedicular access at the thoracic level and a bipedicular for lumbar procedures, mainly for the higher axial load forces at this level, in which we believed that a greater quantity of BFM was required. As for VHR, no significant difference was observed between the two approaches.
Regarding VHR, stability at post-treatment represents an important target. Several studies have shown that after percutaneous kyphoplasty, an important reduction in VBH routinely occurs, probably due to an inhomogeneous BFM distribution. PKP procedure requires balloon inflation inside the VB and successive withdrawal to allow cement injection of cement, causing a partial collapse of the vertebral body, resulting in negative effects on height restoration [5, 17, 18].
Conversely, this event is more common in standard PVP when the fracture remains unstable. In this case, a new PVP intervention is advocated; however, it must be noted that the risk of undesired cement leakage is greatly increased [9, 19–22].
In our series, we did not observe any perceivable difference in VBH between the procedure final control and follow-up assessment, suggesting how the Vessel-X device offers good support for the fractured vertebral body preventing its collapse.
Furthermore, vesselplasty guarantees shorter exposure time to ionizing radiation, as for the first 2 mL of injected BFM, no fluoroscopic control is required. Then, a fluoroscopy examination is necessary for every 0.25 mL of BFM injected until the desired VBH is reached [17].
Limitations
Our study presents some limitations: it is a retrospective single-center study and on relatively small sample size. However, our results are encouraging and, if confirmed, would allow patients with exclusion criteria like interrupted posterior wall to be treated.
CONCLUSION
Vesselplasty technique using Vessel-X may be considered an effective and safe option for the treatment of standard and complex VFs.
Due to its design, Vessel-X guarantees optimal control of BFM distribution with a reduced rate of cement leakage and shorter fluoroscopy time compared to PVP and PKP procedures.
Vessel-X has also shown good clinical results with a significant reduction of NRS and ODI values post-treatment.
However, to further validate these results, prospective randomized trials are necessary.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. S. Masala ― conception of the work, drafting and revising the work; A. Lacchè ― acquisition, analysis, interpretation of data for the work; Ch. Zini ― analysis, interpretation of data for the work; D. Mannatrizio ― drafting and revising the work; S. Marcia ― interpretation of data for the work; M. Bellini, G. Guglielmi ― drafting and revising the work.
About the authors
Salvatore Masala
University of Foggia
Email: salva.masala@tiscali.it
ORCID iD: 0000-0003-0032-7970
MD
Italy, FoggiaAdriano Lacchè
University of Foggia
Email: adrianolacche@gmail.com
ORCID iD: 0000-0003-1782-8624
MD
Italy, FoggiaChiara Zini
University of Foggia
Email: zini.chiara@gmail.com
ORCID iD: 0000-0003-3456-4106
MD
Italy, FoggiaDomenico Mannatrizio
University of Foggia
Email: dr.mannatrizio@gmail.com
ORCID iD: 0000-0003-3365-7132
MD
Italy, FoggiaStefano Marcia
University of Foggia
Email: stemarcia@gmail.com
ORCID iD: 0000-0002-2118-9864
MD
Italy, FoggiaMatteo Bellini
University of Foggia
Email: matteo.bellini@icloud.com
ORCID iD: 0000-0002-1704-6246
MD
Italy, FoggiaGiuseppe Guglielmi
University of Foggia
Author for correspondence.
Email: giuseppe.guglielmi@unifg.it
ORCID iD: 0000-0002-4325-8330
MD, Professor
Italy, FoggiaReferences
- Kushchayev SV, Wiener PC, Teytelboym OM, et al. Percutaneous vertebroplasty: a history of procedure, technology, culture, specialty, and economics. Neuroimaging Clin N Am. 2019;29(4):481–494. doi: 10.1016/j.nic.2019.07.011
- Bornemann R, Koch EM, Wollny M, Pflugmacher R. Treatment options for vertebral fractures an overview of different philosophies and techniques for vertebral augmentation. Eur J Orthop Surg Traumatol. 2014;24(Suppl 1):S131–143. doi: 10.1007/s00590-013-1257-3
- Flors L, Lonjedo E, Leiva-Salinas C, et al. Vesselplasty: a new technical approach to treat symptomatic vertebral compression fractures. AJR Am J Roentgenol. 2009;193(1):218–226. doi: 10.2214/AJR.08.1503
- Tsoumakidou G, Too CW, Koch G, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol. 2017;40(3):331–342. doi: 10.1007/s00270-017-1574-8
- Filippiadis DK, Marcia S, Masala S, et al. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol. 2017;40(12):1815–1823. doi: 10.1007/s00270-017-1779-x
- Diel P, Röder C, Perler G, et al. Radiographic and safety details of vertebral body stenting: results from a multicenter chart review. BMC Musculoskelet Disord. 2013;14:233. doi: 10.1186/1471-2474-14-233
- Vanni D, Galzio R, Kazakova A, et al. Third-generation percutaneous vertebral augmentation systems. J Spine Surg. 2016;2(1):13–20. doi: 10.21037/jss.2016.02.01
- Anselmetti GC, Manca A, Marcia S, et al. Vertebral augmentation with nitinol endoprosthesis: clinical experience in 40 patients with 1-year follow-up. Cardiovasc Intervent Radiol. 2014;37(1):193–202. doi: 10.1007/s00270-013-0623-1
- Zhan Y, Jiang J, Liao H, et al. Risk factors for cement leakage after vertebroplasty or kyphoplasty: a meta-analysis of published evidence. World Neurosurg. 2017;101:633–642. doi: 10.1016/j.wneu.2017.01.124
- Tempesta V, Cannata G, Ferraro G, et al. The new Vessel-X kyphoplasty for vertebral compression fractures: 2-year follow-up of 136 levels. Las Vegas: American Academy of Orthopaedic Surgeons Annual Meeting; 2009.
- McCall T, Cole C, Dailey A. Vertebroplasty and kyphoplasty: a comparative review of efficacy and adverse events. Curr Rev Musculoskelet Med. 2008;1:17–23. doi: 10.1007/s12178-007-9013-0
- Mroz TE, Yamashita T, Davros WJ, Lieberman IH. Radiation exposure to the surgeon and the patient during kyphoplasty. J Spinal Disord Tech. 2008;21(2):96–100. doi: 10.1097/BSD.0b013e31805fe9e1
- Ruiz Santiago F, Santiago Chinchilla A, Guzmán Álvarez L, et al. Comparative review of vertebroplasty and kyphoplasty. World J Radiol. 2014;6(6):329–343. doi: 10.4329/wjr.v6.i6.329
- Hiwatashi A, Yoshiura T, Yamashita K, et al. Morphologic change in vertebral body after percutaneous vertebroplasty: follow-up with MDCT. AJR Am J Roentgenol. 2010;195:W207–W212. doi: 10.2214/AJR.10.4195
- Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech. 2005;18(3):238–242.
- Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25(2):175–180.
- Bambang D. Vesselplasty: a novel concept of percutaneous treatment for stabilization and height restoration of vertebral compression fractures. J Musculoskeletal Res. 2008;11(2):71–79. doi: 10.1142/s0218957708001985
- Zheng Z, Luk KD, Kuang G, et al. Vertebral augmentation with a novel Vessel-X bone void filling container system and bioactive bone cement. Spine (Phila Pa 1976). 2007;32(19):2076–2082. doi: 10.1097/BRS.0b013e3181453f64
- Carlier RY, Gordji H, Mompoint DM, et al. Osteoporotic vertebral collapse: percutaneous vertebroplasty and local kyphosis correction. Radiology. 2004;233(3):891–898. doi: 10.1148/radiol.2333030400
- Chen WJ, Kao YH, Yang SC, et al. Impact of cement leakage into disks on the development of adjacent vertebral compression fractures. J Spinal Disord Tech. 2010;23(1):35–39. doi: 10.1097/BSD.0b013e3181981843
- Komemushi A, Tanigawa N, Kariya S, et al. Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol. 2006;29(4):580–585. doi: 10.1007/s00270-005-0138-5
- Guarnieri G, Masala S, Muto M. Update of vertebral cementoplasty in porotic patients. Interv Neuroradiol. 2015;21(3):372–380. doi: 10.1177/1591019915582364
Supplementary files