肿瘤内分泌学中的多模式数据库: 放射性碘难治性分化型甲状腺癌
- 作者: Rumyantsev P.O.1, Baidak A.G.2
-
隶属关系:
- International Medical Center “SOGAZ-MEDICINE”
- M-Data
- 期: 卷 3, 编号 1 (2022)
- 页面: 86-93
- 栏目: Editorials
- ##submission.dateSubmitted##: 11.03.2022
- ##submission.dateAccepted##: 08.04.2022
- ##submission.datePublished##: 24.04.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/104745
- DOI: https://doi.org/10.17816/DD104745
- ID: 104745
如何引用文章
详细
个性化循证医学和肿瘤学方面的高成就,尤其是在发达国家,与成功开发了多模式数据库“临床”癌症患者登记系统(SEER、NCDB 等)有关。它们是用于开发分析和预后工具的数据矩阵,用于研究诊断特征、疾病的临床过程、对治疗的反应、评估预后因素的影响等。 从医疗数字数据库的角度来看,在做出医疗决策时,数据的冗余和重复并不像信息的不完整或不一致那么重要。本文目的是宣布一个放射性碘难治性分化型甲状腺癌患者的多模式数据库,该数据库本质上是一个现代跨学科数字医疗登记系统。除了流行病学登记的典型人口统计学和疾病学数据外,多模式数据库还考虑了关键的临床和临床旁数据:实验室结果、形态学和仪器研究方法、各种成像方法(超声、CT、MRI、SPECT/CT、PET/计算机断层扫描)。多模式数据库展示了肿瘤分子遗传学谱的结果,其在治疗策略选择中的临床实用性在今天是毫无疑问的。所有这些数据都累积在多模式数据库中,并在执行时间、审查结果(第二意见)上进行标记,同时考虑到可能影响临床过程、治疗反应的标准化定性和定量参数(因素),并发症和结果的发展。
全文:
绪论
在俄罗斯,甲状腺癌的发病率从1998年的每10 万人口4.41人增加到2019年的9.53人,增加了一倍多[1, 2]。这一增长主要是由于广泛使用的超声扫描对乳头状微癌的额外检测。
目前发现的甲状腺癌中约70–80%预后良好,尤其是在早期发现时,这种患者术后不适合碘-131治疗[3]。约三分之一的分化型甲状腺癌(DTC)患者有肿瘤复发和远处转移,平均10%的患者对放射性碘或放射性碘难治性(RR)表现出难治性[4]。
多模式数据库
当今世界,癌症患者登记处(SEER、NCDB等)或多模式数据库(IDB)是研究疾病临床过程特征、治疗反应、预后因素影响评估等的主要工具 [5]。将不同学科的专家整合到一个信息平台上,可以快速沟通,为医疗决策提供专家支持 [6] 。医疗决策的准确性和速度的主要利益相关者是病人,因此有必要让病人作为国内和手术医疗信息(医疗记录、测试、光盘、玻璃等)的提供者参与进来,包括纸质和电子数字格式。从医学在线数据库的角度来看,冗余和重复并不重要,但不完整或不一致的信息对医疗决策至关重要。
为了获得关于罕见疾病患者的综合数据,欧盟罕见病专家委员会(EUCERD——European Union Committee of Experts on Rare Diseases) ,包括罕见癌症联合专家委员会,发病率低于每10 万人口6人,其中包括甲状腺癌[7]。对于当前在俄罗斯的时刻也提出了几个行动-不同级别的医疗保健组织的登记处保护,但没有一个考虑到客户数据关于病人。
放射性碘难治性分化型甲状腺癌患者的多模式数据库
本文旨在普及建立放射性碘难治性分化型甲状腺癌患者多模式数据库(RR DTC IDB)的项目。RR DTC IDB基本上是一个最先进的临床注册,并得到了内分泌外科医生协会as-endo.ru、头脖肿瘤学会(hnonco.ru) 和发展治疗学协会theranostics.ru的支持。
除了流行病学登记所特有的人口学和病理学数据外,正在开发的RR DTC IDB还考虑到相关的临床和辅助临床数据:实验室、形态学和仪器检查方法的结果,以及各种成像技术(超声、CT、MRI、 SPECT/CT、PET/CT)(见表放) 。计划记录RR DTC IDB肿瘤的分子遗传图谱结果( 在手术过程中),其在治疗策略选择中的临床效益在今天是毋庸置疑的。所有这些数据都将积累在RR DTC IDB中,记下实施的时间、审查的结果(第二意见),考虑到可能影响临床过程、对治疗的反应、并发症的发展和结果的标准化定性和定量参数(因素)。
许多研究表明,肿瘤的生长和甲状腺癌的进展与BRAF、RAS和RET基因的体细胞点突变密切相关。这些突变有助于激活丝裂原活化蛋白激酶(MAPK)和磷酸肌醇3-激酶(PI3Ks)的增殖信号通路,这是甲状腺癌发生发展的关键[8]。临床医生应记住,根据俄罗斯联邦卫生部的建议,细胞学结论为Bethesda IV、V和VI的患者可被指派对BRAF、TERT、RAS、RET/PTC、PAX8/PPAR-γ等[9]基因进行分子遗传学研究。
RR DTC IDB项目收集关于BRAF、TERT、RAS、RET/PTC、PAX8/PPAR-γ基因已知突变的分子遗传学检测结果的信息,以便进一步分析。
在创建的RR DTC IDB中,可以使用模板输入记录数据(以便在进一步显示时以一种形式显示数据)、编辑现有记录、添加日期更改和以PDF格式附加文档。
表放射性碘难治性分化型甲状腺癌患者多模式数据库中存储和处理的数据结构
项目 | 变数 |
项目 | 变数 |
人口统计学数据 |
|
病史 |
|
诊断 |
|
原发肿瘤期 |
|
遗传学(肿瘤) |
|
仪器诊断 | 日期
肿瘤动态评估(RECIST):
|
手术治疗 | 进行手术的日期
并发症(手术治疗):
|
放射性碘难治性(RR)与放射性碘疗法(RIT) | 手术日期
放射性药品在甲状腺床投影、颈部区域淋巴结投影、腹膜后淋巴结、肺部投影、骨骼中的分布和积累,其他病理积累的位置 放射性耐药转移存在(记录RR的日期!),病灶的位置和大小,进展 (RECIST 1.1.) 并发症(放射性碘疗法):
|
实验室诊断 | 检查日期
|
实验室诊断 |
|
组织学 | 检查日期
|
激素治疗 | 预约检查日期
|
放射性碘抗性(总结) | RR日期(见放射性碘难治性/放射性碘疗法部分) 碘-131的总活性(包括记录RR,GBq/mcg) |
靶向治疗 | 日期(预约、更正、取消)
|
靶向治疗的不良反应(并发症) | UP登记日期
|
注:TH——促甲状腺激素;Tg——甲状腺球蛋白;TGA——甲状腺球蛋白抗体;fT4——游离四碘甲状腺激素;ASL——局部淋巴结的解剖和手术水平;MO——医疗机构;UP——不良现象。
创建的RR DTC IDB是数字医学发展中的整合项目,解决以下健康问题:
- 多模式数据收集和分析的多学科范式,以病人的操作和循证医疗护理为目标;
- 建立对感兴趣的医生可访问的数字资源,用于核算和分析自己的经验,并在真实的临床实践中提供操作性学院专家支持;
- 多中心科研实践创新数字平台;
- 临床和流行病学研究、放射组学发展、深度机器学习、医学人工智能的矩阵;
- 预测新的RR DTC病例,对其进行验证并选择最佳治疗策略;
- 巩固有效和安全的病人治疗(手术、放疗、激素治疗、靶向治疗)的循证经验。
实际内容
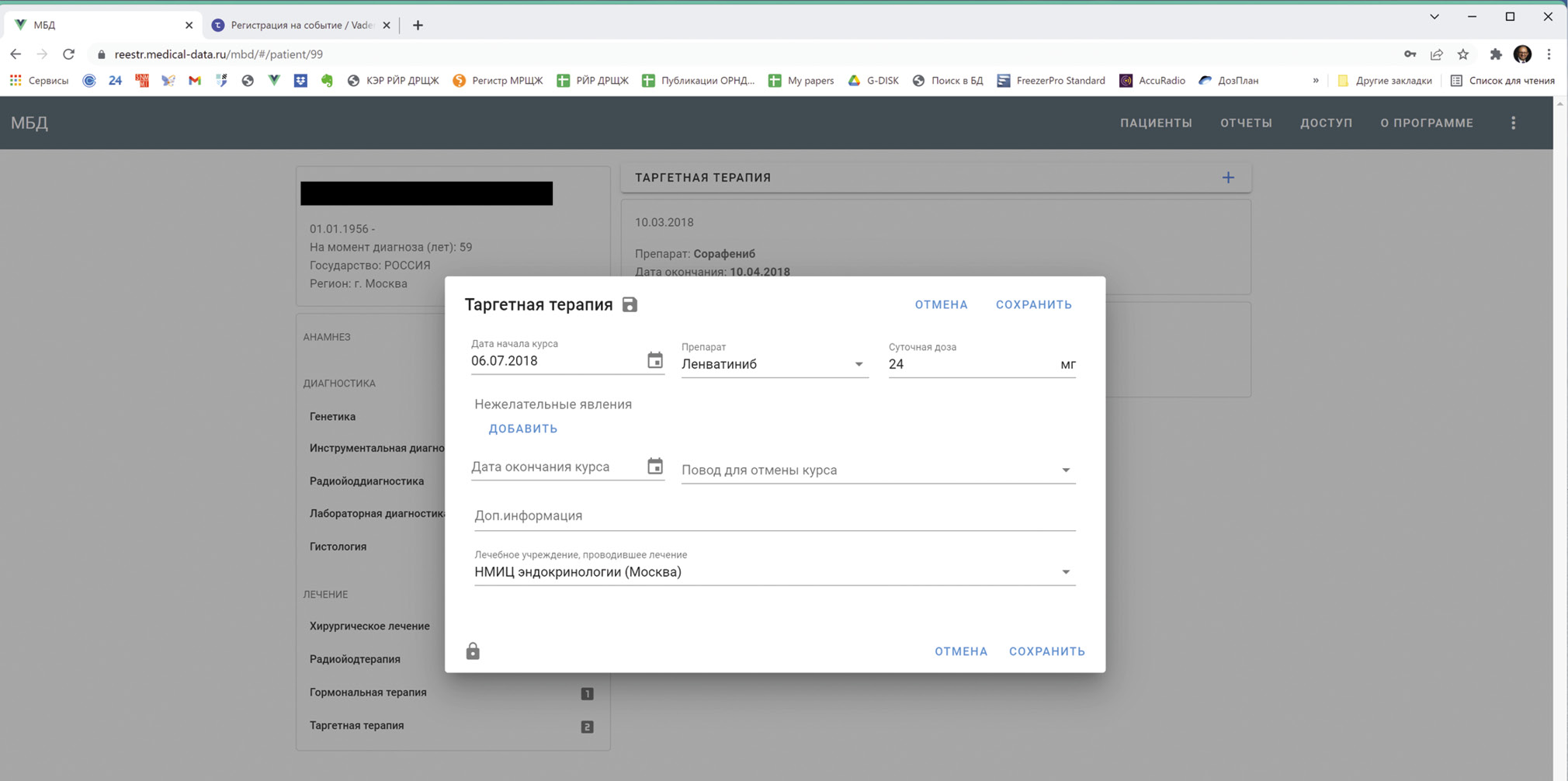
目前,建立RR DTC IDB数据库的项目已在因特网上公布,网址为http://reestr.medical-data.ru/mbd/,广大医学界人士可以查阅和参与。现有版本是11名医生努力的结果,涵盖了俄罗斯联邦48个地区的228名患者的观察数据。所有参与治疗RR DTC的医生(肿瘤学家、外科医生、放射学家、核医学专家、内分泌学家、化疗学家、病理学家、遗传学家)都可以加入该项目。为了加入该项目,开发商提出在网站入口直接在系统中注册。RR DTC IDB数据库的WEB界面(图 1)对任何医生是简单和直观的,因为它是由医生构思和实施的。
图 1针对放射性碘难治性分化型甲状腺癌患者的多模式数据库项目的WEB界面(RR DTC IDB)
需要填写的部分与经典的病史和门诊记录相对应。参与该项目是医生们在工作中拓宽专业视野的机会,学习团队精神,交流管理RR DTC患者的经验,并参与科学和分析工作,在国内外科学医学杂志上共同发表文章。
多模式数据库(临床、实验室、放射学等)的建立促进并推动了多模式合作,这为多中心和国家临床和流行病学循证研究以及改善癌症患者的诊断、治疗和康复技术奠定了基础。这与神经内分泌肿瘤的情况特别相关,例如,这些肿瘤具有高度的侵略性,对标准治疗难有效果。
作者简介
Pavel O. Rumyantsev
International Medical Center “SOGAZ-MEDICINE”
Email: pavelrum@gmail.com
ORCID iD: 0000-0002-7721-634X
SPIN 代码: 7085-7976
MD, Dr.Sci. (Med), Deputy Director of Oncoendocrine Institute
俄罗斯联邦, 8, Malaya Konyushennaya, Saints Peterburg, 191186Andrey G. Baidak
M-Data
编辑信件的主要联系方式.
Email: baidak@medical-data.ru
俄罗斯联邦, Orel
参考
- Chissov VI, Starinsky VV, Petrova GV. Malignant neoplasms in Russia since 2008 (morbidity and mortality). Moscow: P.A. Herzen Federal State Medical Research Institute of Rosmedtechnology; 2010. 256 p. (In Russ).
- Kaprin AD, Starinsky VV, Petrova GV. The state of oncological care for the population of Russia in 2019. Moscow: P.A. Herzen Moscow State Medical Research Institute — branch of the FSBI NMIC of Radiology of the Ministry of Health of Russia,2020. 236 p. (In Russ).
- Pacini F, Fuhrer D, Elisei R, et al. 2022 ETA Consensus Statement: what are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur Thyroid J. 2022;11(1):e210046. doi: 10.1530/ETJ-21-0046
- Rumyantsev PO, Fomin DK, Rumyantseva UV. Criteria of resistance of highly differentiated thyroid cancer to therapy with radioactive iodine. Tumors Head Neck. 2014;(3):4–9. (In Russ).
- Rumyantsev PO, Slashchuk KY, Korenev SV, et al. Interdisciplinary data bank in oncoendocrinology. Medullary thyroid cancer and syndromes of multiple endocrine neoplasia type 2. Endocrine Sur. 2019;13(3):105–117. (In Russ). doi: 10.14341/serg11270
- Almazov AA, Rumyantsev PO, Kupreev PP, et al. Medical decision support systems; analysis of multimodal data, the difference between “human” and “machine” approaches, social problems of collection and turnover of biomedical data. Doctor Information Technol. 2020;(2):28–35. (In Russ). doi: 10.37690/1811-0193-2020-2-28-35
- Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–2511. doi: 10.1016/j.ejca.2011.08.008
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856–1883. doi: 10.1093/annonc/mdz400
- Clinical recommendations “Differentiated thyroid cancer”. Moscow; 2020. 47 p. (In Russ).