糖皮质激素治疗新型冠状病毒感染罕见局限性缺血性坏死
- 作者: Gonchar A.P.1, Blokhin I.A.1, Shumskaya Y.F.1,2
-
隶属关系:
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- The First Sechenov Moscow State Medical University (Sechenov University)
- 期: 卷 3, 编号 4 (2022)
- 页面: 384-392
- 栏目: 临床病例及临床病例的系列
- ##submission.dateSubmitted##: 31.08.2022
- ##submission.dateAccepted##: 11.11.2022
- ##submission.datePublished##: 30.12.2022
- URL: https://jdigitaldiagnostics.com/DD/article/view/110718
- DOI: https://doi.org/10.17816/DD110718
- ID: 110718
如何引用文章
详细
糖皮质激素治疗新型冠状病毒感染引起的骨结构缺血性坏死是一种相当常见的治疗并发症,其中最常见的是股骨头病变。对于预防关节炎和其他并发症,及时检测缺血性坏死是很重要的。
这项研究展示了一名54岁的患者的临床案例,她因新冠状病毒感染住院,并在发病后两周内抱怨双膝关节疼痛。磁共振成像扫描显示,形成膝关节的骨骼在两侧都有明显的血管性坏死。使用非甾体抗炎药和双磷酸盐骨吸收抑制剂的保守治疗有显著的积极效果。3个月后复查时,没有疼痛,但膝关节的活动仍有轻微限制。两个膝关节的磁共振成像显示,先前确定的变化明显减少。
糖皮质激素的副作用(糖耐量受损、血压升高、心动过速、胃肠道侵蚀性溃疡、睡眠障碍等)广为人知,但由类固醇引起的膝关节骨结构的骨坏死却很少引起临床医生的注意。这个临床病例强调了骨坏死发病机制的复杂性,并展示了皮质类固醇治疗的广泛并发症。
全文:
研究的现实意义
2019年12月在中国武汉首次发现的一种新型冠状病毒感染已引起全球大流行。在新型冠状病毒感染的治疗中,糖皮质激素(GC)的应用在发病机制上是合理及广泛的[1]。然而,众所周知,不仅是密集使用,甚至是单次使用都会引起血管性坏死的发生[2]。文献描述了许多接受糖皮质激素治疗的患者发生股骨头缺血性坏死的病 例[3, 4] ,然而,其他部位的骨坏死,尤其是膝关节,仅在个别病例中出现[5]。此类病理的早期诊断对于预防关节炎和其他并发症的发展极为重要[6]。
在这篇文章中,我们描述了一个在冠状病毒感染的GC治疗过程中出现的双膝关节骨结构无血管坏死的病例。
病例报告
关于患者
一名54岁的妇女主诉剧烈咳嗽和连续6天发烧高达39.5ºC,因新型冠状病毒感染住院。胸部CT扫描显示超过30%的肺体积受损。确定了SARS-CoV-2 RNA聚合酶链反应的阳性结果。患者的躯体病史没有收到影响。
住院期间,患者接受了以下剂量的治疗:地塞米松肠外给药5天,每日剂量为20毫克,停药2天,再继续给药5天,剂量为12毫克,以及抗凝血剂疗法和抗分泌治疗。
发病第15天,膝关节出现剧烈疼痛,活动明显受限,持续至夜间; 关节触诊疼痛。患者于第17天出院,由于膝关节疼痛,建议服用非甾体类抗炎药。
住院后1.5个月,由于持续主诉膝关节疼痛和活动受限,患者被转诊进行双膝关节磁共振成 像(MRI)。
仪器检查的结果
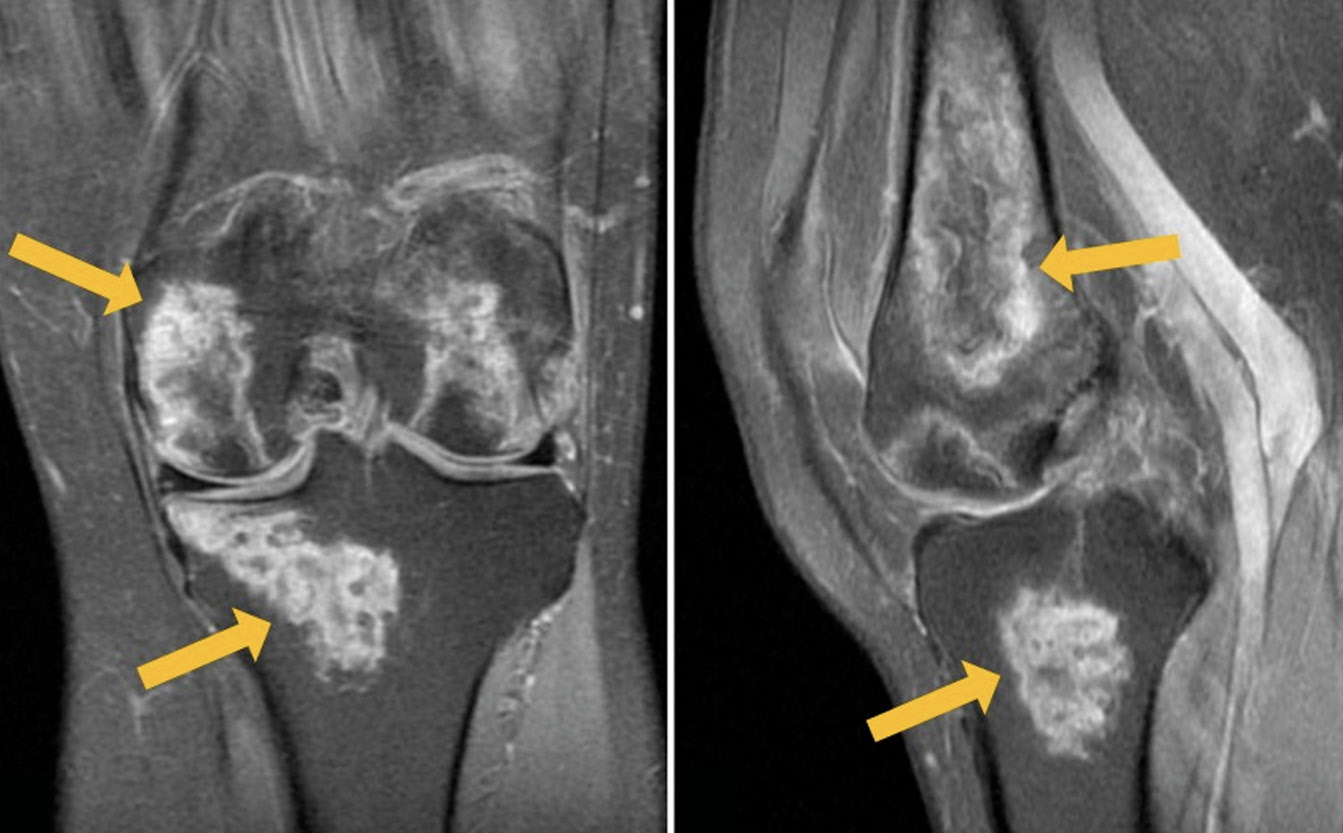
根据左膝关节MRI:骨干远端、股骨髁(包括关节面)、髌骨均有不均匀高信号病变区在PD加权(质子加权)图像上抑制来自脂肪组织的信号,T1 加权图像(T1-WI)上的低/等信号,不规则(“地理”)形状,在其中央部分,可以看到具有黄骨髓信号特征的区域(图1)。右膝关节的MRI检查显示两个股骨髁的骨髓有类似变化的区域,并扩散到远端干骨后端和外侧骨髁的关节面,以及髌骨。沿着一些显露病变区的外围,在短距离内可见“双线”症状(图2)。
图1。左膝关节的初级磁共振成像:PD-WI在冠状面(a)和矢状面 (b)中具有来自脂肪组织的信号抑制,矢状面中的T1-WI(c)。箭头表示股骨和胫骨髁的异质、不规则形状(“地理”)MRI信号形式的骨髓水肿区域。
图2。右膝关节的初级磁共振成像:PD-WI在冠状面(a)和矢状面(b)中具有来自脂肪组织的信号抑制,矢状面中的T1-WI(c)。粗箭头表示股骨髁和髌骨的异质、不规则形状(“地理”)MRI信号形式的骨髓水肿区域;细箭头表示PD-WI上内部高信号(肉芽组织)和外部低信号(骨硬化)线形式的“双线”症状。
由于现有的病史和核磁共振扫描发现的变化,诊断为“形成两侧膝关节的骨骼缺血性坏死”。
诊断、治疗
根据MRI结果,结合病史和临床表现,诊断为双膝关节股骨和胫骨骨髁缺血性坏死。在这方面,保守疗法(物理疗法)规定如下:使用含有非甾体类抗炎成分的凝胶进行磁疗和超声透入疗法,疼痛情况下,片剂形式的非甾体类抗炎药,维生素D制剂,双磷酸盐骨吸收抑制剂。
在3个月后的治疗背景下,出现了明显的积极趋势:疼痛综合症停止了;膝关节的运动受到轻微限制(无法深蹲)。
双膝关节的重复MRI显示出积极的趋势:之前发现的变化不太明显(图 3、4)。
图3。左膝关节的重复磁共振成像:PD-WI在冠状面(a)和矢状面(b)中具有来自脂肪组织的信号抑制,矢状面中的T1-WI(c)。粗箭头表示股骨髁和髌骨的异质、不规则形状(“地理”)MRI信号形式的骨髓水肿区域; 细箭头表示PD-WI上内部高信号(肉芽组织)和外部低信号(骨硬化)线形式的“双线”症状。
讨论
新型冠状病毒感染患者骨坏死的患病率差异很大——从5%到58%[7],其中股骨头病变更为常见,而膝关节和其他骨骼的骨结构损伤则较少见。
在膝关节骨骼结构的继发性骨坏死中,即无血管性坏死,通常两个股骨髁都受到影响,如本临床病例;反过来,原发性骨坏死的特点是仅对一个骨髁造成损伤[8]。
发生缺血性坏死的原因包括糖皮质激素、肾脏疾病、血液病的治疗。 一些作者注意到用于治疗冠状病毒感染的药物(例如洛匹那韦和利托那韦)对骨坏死发展的影响[9]。在所提供的案例中,这种病理状况很可能是在使用糖皮质激素治疗冠状病毒感染期间发生的。
GC作为治疗新型冠状病毒感染的重要制剂,是发生缺血性坏死的独立危险因素。同时,此类患者发生骨坏死的发病机制尚不完全清楚:除了GC治疗以外,血管血栓形成、脂肪细胞肥大、脂肪栓塞、高凝、内皮破坏和白细胞聚集均可成为独立原因[7,10]。然而,文献中尚无因上述因素而发生骨坏死的病例[7,9]。
目前对GC的使用期限或增加骨坏死风险的剂量没有明确的意见。然而,一系列研究表明,控制GC的累积剂量作为病理发展的重要因素非常重要 [11]。例如,一些研究确定,膝关节骨结构的骨坏死是在泼尼松龙的累积剂量为1.012-6.562克时发生的[12,13],而在其他临床病例中,泼尼松龙的累积剂量为0.9-1.413克,平均为1.156克[14]。在我们描述的临床病例中,地塞米松的使用剂量为20毫克/天,随后减至12毫克。
S.R. Agarwala及合著者[14]描述了一名20岁女性的甲基强的松龙用药15天后出现无血管性坏死的病例,发病第25天出现膝关节疼痛,MRI显示双侧髁状突和髌骨有病变;一名感染新感染冠状病毒的16岁男性,在使用甲基强的松龙和地塞米松治疗4个月后,出现双髋和右膝疼痛,持续19天。在这个病例中,地塞米松使用了10天,间断2天,15天后(从GC治疗开始的第9天)首次出现膝关节疼痛的主诉,这与上面第一位患者的临床例子相似,尽管我们患者的年龄明显不同。
另一个临床病例描述了一名78岁女性右膝关节缺血性坏死的发展,该女性有双侧膝关节病史,左侧更为明显,并伴有心血管疾病、肥胖[15]。治疗包括抗菌药物、羟氯喹、抗病毒药物(洛匹那韦、利托那韦)和氧疗。出院2周后,患者发现右膝关节疼痛加重。同时,由于支气管痉挛的出现,进行了9天的GC治疗。7天后,右膝关节出现局部水肿。MRI显示右股骨内侧髁骨坏死迹象。在这种情况下,仅GC对血管性坏死的影响是不明确的:同时存在的疾病也是骨坏死的一个风险因素。然而,冠状病毒感染和血管性坏死发展之间的时间很短,这表明GC给药的影响。在我们研究的的临床病例中,除了服用GC以外,患者没有任何血管性坏死的危险因素,但是,如上例所示,在GC治疗期间关节痛的发展相当迅速。
另一方面,A. Sulewski及合著者[16]的研究结果表明,缺乏关于GC对骨坏死发展的直接影响的数据。这项工作包括对10名确诊冠状病毒感染和无血管性坏死迹象的患者进行分析。患者的平均年龄为61岁。尽管10名患者中只有4名接受了包括GC的治疗,但他们中的每一个在发病后平均14天后都出现了无血管性坏死的迹象。W. Li等人[17]在一项荟萃分析中得到了类似的数据,证实了新型冠状病毒感染患者无血管性坏死的多因素发病机制理论。作者将血管紧张素转换酶2的缺乏列为这些患者发生无血管性坏死的一个可能因素,这可能导致骨质破坏以及血管血栓形成,就像GC治疗的骨坏死机制那样。因此,对于新型冠状病毒感染患者发生无血管性坏死的病因和发生机制,目前尚无明确意见。
无论是在我们介绍的临床病例还是在国外作者的研究著作中,对无血管性坏死的治疗均以保守治疗为主,主要目的是减轻疼痛,减缓骨坏死的进展,预防骨折和关节炎。然而,还没有描述普遍接受的治疗方案[18]。反过来,一系列研究人员证实,使用双膦酸盐的综合治疗可有效治疗骨坏死,包括在早期阶段,我们的临床病例证实了这一点[19,20]。
因此,及早发现因新冠病毒感染而发生无血管性坏死的风险增加的患者,对于预防关节炎和其他并发症的发生极为重要。
结论
我们描述了一例因COVID-19的GC给药而通过MRI检测到的双侧膝关节形成骨的无血管性坏死的临床病例。使用GS的副作用,例如葡萄糖耐量降低、血压升高、心动过速、胃肠道的侵蚀性和溃疡性损伤、睡眠障碍,是广为人知的。然而,由GS引起的膝关节骨结构无血管性坏死很少引起临床医生的注意。这个临床病例强调了骨坏死发病机制的复杂性,并展示了GS治疗的广泛并发症。
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. A.P. Gonchar — concept and design of the paper; A.P. Gonchar — data collection and analysis; Yu.F. Shumskaya — data interpretation; I.A. Blokhin — approval of the final version of the paper.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information within the manuscript in Digital Diagnostics Journal.
Acknowledgments. The authors are thankful to Victoria Alexandrovna Matkova for her contribution to the publication.
作者简介
Anna P. Gonchar
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: a.gonchar@npcmr.ru
ORCID iD: 0000-0001-5161-6540
SPIN 代码: 3513-9531
俄罗斯联邦, Moscow
Ivan A. Blokhin
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: i.blokhin@npcmr.ru
ORCID iD: 0000-0002-2681-9378
SPIN 代码: 3306-1387
俄罗斯联邦, Moscow
Yuliya F. Shumskaya
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies; The First Sechenov Moscow State Medical University (Sechenov University)
编辑信件的主要联系方式.
Email: yu.shumskaia@npcmr.ru
ORCID iD: 0000-0002-8521-4045
SPIN 代码: 3164-5518
俄罗斯联邦, Moscow; Moscow
参考
- Temporary guidelines «Prevention, diagnosis and treatment of new coronavirus infection (COVID-19)». Version 3 (March 3, 2020) (approved by the Ministry of Health of the Russian Federation). (In Russ). Available from: https://www.garant.ru/products/ipo/prime/doc/73647088/. Accessed: 15.07.2022.
- Ali RS, Al-Sudani H, Tan IJ. Osteonecrosis of bilateral distal femurs in a pregnant patient following antenatal betamethasone. Cureus. 2022;14(3):e22735. doi: 10.7759/cureus.22735
- Zhang S, Wang C, Shi L, Xue Q. Beware of steroid-induced avascular necrosis of the femoral head in the treatment of COVID-19-experience and lessons from the SARS epidemic. Drug Des Devel Ther. 2021;15:983–995. doi: 10.2147/DDDT.S298691
- Agarwala SR, Vijayvargiya M, Pandey P. Avascular necrosis as a part of ‘long COVID-19’. BMJ Case Rep. 2021;14(7):e242101. doi: 10.1136/bcr-2021-242101
- Takao M, Sugano N, Nishii T, et al. Spontaneous regression of steroid-related osteonecrosis of the knee. Clin Orthop Relat Res. 2006;452:210–215. doi: 10.1097/01.blo.0000229278.51323.08
- Hines JT, Jo WL, Cui Q, et al. Osteonecrosis of the femoral head: an updated review of arco on pathogenesis, staging and treatment. J Korean Med Sci. 2021;36(24):e177. doi: 10.3346/jkms.2021.36.e177
- Disser NP, De Micheli AJ, Schonk MM, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102(14):1197–1204. doi: 10.2106/JBJS.20.00847
- Karim AR, Cherian JJ, Jauregui JJ, et al. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3(1):6. doi: 10.3978/j.issn.2305-5839.2014.11.13
- Patel MS, Gutman MJ, Abboud JA. Orthopaedic considerations following COVID-19: lessons from the 2003 SARS outbreak. JBJS Rev. 2020;8(7):e2000052. doi: 10.2106/JBJS.RVW.20.00052
- Kerachian MA, Séguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114(3-5):121–128. doi: 10.1016/j.jsbmb.2009.02.007
- Chan KL, Mok CC. Glucocorticoid-induced avascular bone necrosis: diagnosis and management. Open Orthop J. 2012;6:449–457. doi: 10.2174/1874325001206010449
- Powell C, Chang C, Naguwa SM, et al. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9(11):721–743. doi: 10.1016/j.autrev.2010.06.007
- Takeda H, Nishise S, Fujishima S, et al. Osteonecrosis of the lateral femoral condyle in a patient with ulcerative colitis: report of a case. Clin J Gastroenterol. 2008;1(3):93–96. doi: 10.1007/s12328-008-0015-2
- Agarwala SR, Vijayvargiya M, Sawant T. Secondary osteonecrosis of the knee as a part of long COVID-19 syndrome: a case series. BMJ Case Rep. 2022;15(3):e248583. doi: 10.1136/bcr-2021-248583
- Angulo-Ardoy M, Ureña-Aguilera Á. Knee osteonecrosis after COVID-19. Fam Pract. 2021;38(Suppl 1):i45–i47. doi: 10.1093/fampra/cmab063
- Sulewski A, Sieroń D, Szyluk K, et al. Avascular necrosis bone complication after active COVID-19 infection: preliminary results. Medicina (Kaunas). 2021;57(12):1311. doi: 10.3390/medicina57121311
- Li W, Huang Z, Tan B, et al. General recommendation for assessment and management on the risk of glucocorticoid-induced osteonecrosis in patients with COVID-19. J Orthop Translat. 2021;31:1–9. doi: 10.1016/j.jot.2021.09.005
- Mont MA, Baumgarten KM, Rifai A, et al. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82(9):1279–1290. doi: 10.2106/00004623-200009000-00008
- Agarwala S, Sharoff L, Jagani N. Effect of zoledronic acid and alendronate on bone edema and pain in spontaneous osteonecrosis of the knee: a new paradigm in the medical management. Rev Bras Ortop (Sao Paulo). 2020;55(5):543–550. doi: 10.1016/j.rboe.2017.12.008
- Jureus J, Lindstrand A, Geijer M, et al. Treatment of spontaneous osteonecrosis of the knee (SPONK) by a bisphosphonate. Acta Orthop. 2012;83(5):511–514. doi: 10.3109/17453674.2012.729184
补充文件