一名6岁儿童的左室心肌致密化不全和左心室动脉瘤
- 作者: Dautov T.B.1, Kaliyev B.B.1, Yerekesh B.T.2
-
隶属关系:
- «University Medical Center» Corporate Fund
- National Research Cardiac Surgery Center
- 期: 卷 4, 编号 4 (2023)
- 页面: 625-632
- 栏目: 临床病例及临床病例的系列
- ##submission.dateSubmitted##: 17.07.2023
- ##submission.dateAccepted##: 06.09.2023
- ##submission.datePublished##: 15.12.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/551837
- DOI: https://doi.org/10.17816/DD551837
- ID: 551837
如何引用文章
详细
左室心肌致密化不全是一种罕见的心肌病。它通常伴有心室动脉瘤。本文描述了一个6岁女孩的临床病例。这名女孩因主诉在体育活动时身体不适而被送进本诊所。超声心动图检查显示了左心室壁有明显的小梁,侧壁基底部有隆起。这些症状与左心室动脉瘤和左室心肌致密化不全相符。心脏磁共振成像显示了,非致密层与致密层的比例为2.6:1,证实了存在左室心肌致密化不全。此外,还发现了收缩功能障碍和伴有心肌瘢痕形成的左心室动脉瘤。冠状动脉造影排除了冠状动脉病变。在这种情况下,我们可以认为心内膜瘢痕形成的性质是非致密心肌层微循环障碍的结果。
全文:
绪论
左室心肌致密化不全是一种罕见的心肌病,是由于胚胎发育过程中心肌致密性形成受损所致。其特征是心肌纤维过度小梁化,并形成深的小梁间 袋[1]。临床表现从无症状到心脏异常以及心力衰竭、心律失常和全身性血栓栓塞[2]。文献中仅有零星报道左心室动脉瘤加重左室心肌致密化不全[3],尤其是在儿童患者中。临床上,大多数左心室动脉瘤无症状,但在极少数情况下,它们可能是导致心律失 常(18.4%)、栓塞事件(5.4%)、心肌破裂(4%)、充血性心力衰竭(21.5%)和心绞痛的原因[4]。
近年来,就左室心肌致密化不全的发展而言,有两种主要假说:胚胎发育过程紊乱和分子遗传机制。起初,人们认为在异常的胚胎形态发生过程中,心肌细胞压实不足导致心肌和小梁间隙的过度小梁化。然而,最近分子遗传学研究方法的改进发现了越来越多与左室心肌致密化不全发生有关的基因。其中大部分是肉瘤蛋白和离子通道基因以及线粒体基因,而肉瘤蛋白基因最常参与疾病的发病机制[5]。在成人中,孤立性左室心肌致密化不全的发病率从0.01%到0.3%不等[6]。美国心脏协会将左室心肌致密化不全归为原发性遗传性心肌病,而欧洲心脏病学会则将其归为未分类的心肌 病[7]。
病例介绍
一名6岁女孩因主诉体力不支而被送入我院。
病史
她在胎儿发育第26周时被诊断出患有渗出性心包炎。15个月大时,超声心动图检查发现了心包空间有积液,体积高达600ml,于是进行了心包穿刺术,结果是诊断为心包积血。入院前,女孩被诊断为心力衰竭,左心室射血分数轻微下 降(53%)。
观察
入院时体温为36.6℃,血氧饱和度(SpO2)为98%,呼吸频率为每分钟23次。心音低沉,有节律的,无器质性杂音。血压为115/83mmHg,心率为每分钟110次。
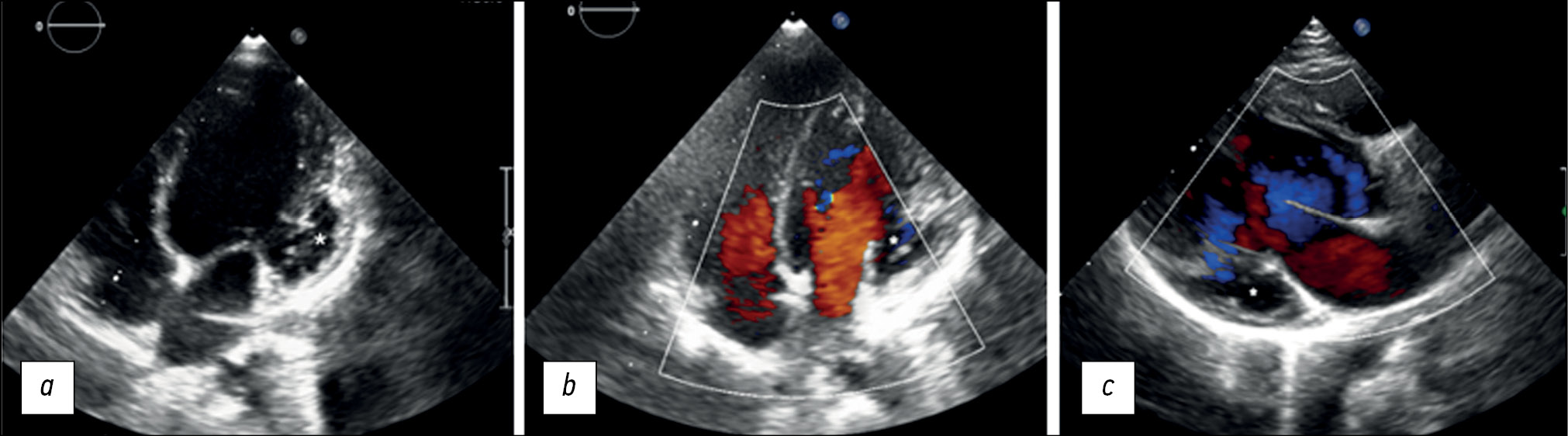
住院时,超声心动图检查显示了左心室扩张,左心室心顶部和侧壁小梁化增大,左心室基底外侧水平出现动脉瘤形式的囊状肿胀,可能是深部小梁(图1,a–c)。此外,左心室收缩功能下降,射血分数为48%,三尖瓣、二尖瓣和肺动脉瓣反流程度轻微。
图1。一名患有心肌致密不全和左心室动脉瘤的6岁患者的经胸超声心动图(心率传感器,二维扫描):a——灰阶模式下的心尖四腔投影,可见左室壁隆起和心肌致密不全,心室与动脉瘤相连(星形);b——彩超模式下的心尖四腔投影,可见一个大尺寸的无气囊性肿块(动脉瘤以星形表示),左心室顶部和侧壁区域存在明显的小梁化;c——彩超模式下的三腔投影,可见一个动脉瘤(星形)、左心室的入口和出口。
超声心动图检查显示了,主要心律为窦性心律。24小时动态心电图监测显示为窦性心律,平均心率为109次/分,最低64次/分,最高173次/分,无超过2秒以上的停顿,无慢快综合征。
为排除先天性心脏病,对心脏进行了对比度增 强(钆布醇,2.0ml)的磁共振成像(MRI)。两个心室的收缩功能均下降:左心室射血分数为41%,右心室射血分数为45%。左心室心肌总质量为54.6g,其中致密化不全的心肌为14.4g,占总质量的26.3%。心脏磁共振成像显示了心肌瘢痕的迹象、左心室基底前壁和侧壁的囊状隆起、心肌致密化不全(非致密层与致密层的比例为2.6:1)、左心室扩张。在延迟断层扫描图片中,观察到造影剂积聚(在第一、第五和第六节 段)(图2,a–e)。
图2。一名患有心肌致密化不全和左心室动脉瘤的6岁患者的心脏磁共振成像。扫描是在1.5-T磁共振断层成像仪(MAGNETOM Avanto,Siemens Healthcare,德国)上进行的,扫描过程中需要憋住呼吸:a——注射造影剂前的短轴双腔图像显示清晰的左心室侧壁隆起(星形)和局部性心肌变薄(箭头)。回波时间(Time of echo,TE)为1.5ms,重复时间(Repetition time, TR)为42ms;b——注射造影剂前的长轴四腔图像显示左心室侧壁基底部的动脉瘤(星形)和心肌致密化不全(非致密层与致密层的比例为2.6:1)。回波时间(Time of echo,TE)为1.5ms,重复时间(Repetition time, TR)为42ms;c——注射造影剂后的长轴四腔图像显示,左心室前外侧壁基底部出现钆晚期增强(箭头),动脉瘤(星形)就位于此处。回波时间(Time of echo,TE)为1.5ms,重复时间(Repetition time, TR)为700ms;d——注射造影剂后的短轴两腔图像显示造影剂在第一、第五和第六节段的积聚情况。回波时间(Time of echo,TE)为1.5ms,重复时间(Repetition time, TR)为2000ms;e——长轴两腔图像显示左心室前壁基底部的钆晚期增强。回波时间(Time of echo,TE)为1.4ms,重复时间(Repetition time, TR)为700ms。
诊断
心脏超声心动图检查和磁共振成像显示了,有左室心肌致密化不全和动脉瘤。于是决定进行冠状动脉造影,以评估冠状动脉血管的状况,结果没有发现冠状动脉异常的迹象(图4,a–b)。
图3。心肌致密化不全伴左心室薄壁动脉瘤。动脉瘤在收缩期从左心室前壁的基底部隆起。箭头表示血流方向。Ao——主动脉;LA——左心房;LV——左心室;NCM——左室心肌致密化不全;RA——右心房;RV——右心室;aneurism——动脉瘤。
图4。一名患有心肌致密化不全和左心室动脉瘤的6岁患者的冠状动脉造影。冠状动脉造影使用一根4 Fr内导管和两根5 Fr导管。a和b——冠状动脉未发现任何变化,左冠状动脉供血类型已确定。RCA——右冠状动脉;LAD——左冠状动脉前降支;Cx——冠状动脉回旋支;D1——对角支;RV branch——右冠状动脉分支;AI——中间支。
根据这些检查结果,诊断出左室心肌致密化不全并发动脉瘤。
考虑到检查结果、体重增加、运动耐量保持不变以及氨基末端脑利钠肽前体NT-proBNP浓度为43.60pg/mL,患者被转诊至居住地医院接受进一步观察,并开具药物治疗处方。
治疗
由于有关该病症疗法的数据有限,建议根据当前针对具体病例的建议治疗临床并发症。对于有血栓栓塞、心房颤动和/或收缩功能障碍(左心室射血分数<40%)病史的左室心肌致密化不全患者,由于深小梁间袋和血流减慢导致血栓风险增加,建议进行抗凝治疗[6]。因此,即使没有收缩功能障碍或心房颤动,左室心肌致密化不全和伴发左心室动脉瘤的患者也有必要接受抗凝治疗。不过,也有患者接受更彻底治疗的报道,包括动脉瘤切除手术,以防止动脉瘤壁的张力和破裂以及血栓风险。此外,切除左心室动脉瘤纤维组织有助于防止心律失常的发生,重塑心室有助于改善心力衰竭的症状。手术干预被认为是一种完善的治疗方法,可与最佳药物治疗同时使用[8]。
讨论
左室心肌致密化不全的诊断主要基于解剖学特征的成像结果。虽然左室心肌致密化不全没有公认的定义,但最常考虑的超声心动图标准如下:
a) 双层心肌在收缩末期有多个明显的小梁;
b) 非致密层与致密层的比例大于2;
c) 彩超模式下可观察到小梁间隙的血流彩色成像,并与左心室腔相通;
d) 无并发心脏异常。
典型的三联并发症包括心力衰竭、室性心律失常和全身性栓塞事件[9]。
我们遇到了一例左室心肌致密化不全合并因微循环障碍导致的左心室动脉瘤的罕见病例。以我们的患者为例,研究表明了,在心脏磁共振成像中,舒张末期心肌非致密层与致密层最大厚度的比例大于2.3,以及左心室小梁心肌质量比左心室总质量大25%是诊断左室心肌致密化不全的关键[10]。
晚期钆对比剂增强显示了,动脉瘤壁有瘢痕。动脉瘤的特点是与心室腔的连接处较宽(图3),而憩室的形状通常较长,颈部较窄。动脉瘤最常见于左心室顶部(28%)和二尖瓣附近的瓣周区域(49%)。左心室动脉瘤通常发生在急性心肌梗塞伴有心肌收缩期隆起并瘢痕形成后。在不了解患者病史和冠状动脉造影结果的情况下,后天性左心室动脉瘤很难与先天性动脉瘤区分开来[11]。然而,微循环障碍被认为是左室心肌致密化不全时发生动脉瘤和瘢痕形成的病因[12]。
根据冠状动脉造影的结果,我们患者的动脉瘤与冠状动脉疾病无关。在我们的病例中,动脉瘤似乎是后天形成的,因为之前的超声心动图检查显示射血分数大于50%,随后当心力衰竭症状开始出现时,射血分数开始下降。左心室动脉瘤的并发症包括动脉瘤内血栓、心输出量变化和动脉瘤破裂。
结论
心肌致密化不全合并左心室动脉瘤是一种极为罕见的心肌病。超声心动图检查、心脏磁共振成像和冠状动脉造影是检测左室心肌致密化不全成分的最有效方法。无论症状的严重程度如何,手术干预可与药物治疗结合。
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Dautov TB ― contribution to the study concept, approved the final version of the work; Kaliyev BB ― revising and editing the manuscript; Yerekesh BT ― data sources collection and analysis, preparation of the manuscript.
Consent for publication. Written consent was obtained from the patient’s legal represantatives for publication of relevant medical information and all of accompanying images within the manuscript.
作者简介
Tairkhan B. Dautov
«University Medical Center» Corporate Fund
Email: tairkhan.dautov@mail.ru
ORCID iD: 0000-0002-5267-0108
SPIN 代码: 8632-6605
MD, Dr. Sci. (Med.), Assistant Professor
哈萨克斯坦, AstanaBauyrzhan B. Kaliyev
«University Medical Center» Corporate Fund
编辑信件的主要联系方式.
Email: baur233113@mail.ru
ORCID iD: 0000-0003-4825-749X
SPIN 代码: 7315-9129
哈萨克斯坦, Astana
Bibissara T. Yerekesh
National Research Cardiac Surgery Center
Email: bibisara_97@mail.ru
ORCID iD: 0009-0002-0185-3278
哈萨克斯坦, Astana
参考
- Srivastava S, Yavari M, Al-Abcha A, Banga S, Abela G. Ventricular non-compaction review. Heart Failure Reviews. 2022;27(4):1063–1076. doi: 10.1007/s10741-021-10128-3
- Petersen SE, Jensen B, Aung N, et al. Excessive Trabeculation of the Left Ventricle. JACC: Cardiovascular Imaging Expert Panel Paper. 2023;16(3):408–425. doi: 10.1016/j.jcmg.2022.12.026
- Catalano MA, Hemli JM, Lasic Z, Patel NC. Repair of left ventricular aneurysm in the setting of noncompaction. Journal of Cardiology Cases. 2022;25(6):416–419. doi: 10.1016/j.jccase.2022.01.008
- Tilahun T, Kedir E, Eshetu B. Fatal Left Ventricular Aneurysm in a 13 Years Old Male Child: A Case Report. Ethiopian journal of health sciences. 2021;31(4):903–906. doi: 10.4314/ejhs.v31i4.26
- Tian S, Liang H, Li X, et al. A novel mutation in the TTN gene resulted in left ventricular noncompaction: a case report and literature review. BMC Cardiovascular Disorders. 2023;23(1):352. doi: 10.1186/s12872-023-03382-w
- Yakabe D, Matsushima S, Uchino S, et al. Left Ventricular Noncompaction with Multiple Thrombi in Apical Aneurysm. Internal Medicine. 2020;59(3):377–381. doi: 10.2169/internalmedicine.3489-19
- Gerecke BJ, Engberding R. Noncompaction Cardiomyopathy-History and Current Knowledge for Clinical Practice. Journal of Clinical Medicine. 2021;10(11):2457. doi: 10.3390/jcm10112457
- Daprati A, Sassi CG, Garatti A, Saitto G, Menicanti L. Congenital left ventricular aneurysm with myocardial noncompaction pattern. Asian Cardiovascular and Thoracic Annals. 2020;28(8):504–506. doi: 10.1177/0218492320949833
- Ogah OS, Iyawe EP, Orimolade OA, et al. Left ventricular noncompaction in Ibadan, Nigeria. The Egyptian Heart Journal. 2023;75(1):69. doi: 10.1186/s43044-023-00396-9
- Gaižauskienė K, Glembockytė G, Glaveckaite S, Valevičienė N. Magnetic resonance diagnostic criteria of non-compaction cardiomyopathy: new diagnostic criteria still needed? Seminars in Cardiovascular Medicine. 2023;29(1):1–13. doi: 10.2478/semcard-2022-0003
- Ohlow MA. Congenital left ventricular aneurysms and diverticula: an entity in search of an identity. Journal of Geriatric Cardiology. 2017;14(12):750–762. doi: 10.11909/j.issn.1671-5411.2017.12.005
- Siripornpitak S, Khositseth A, Sriprachyakul A. Left Ventricular Non-compaction with Ventricular Aneurysms. Journal of Cardiovascular Imaging. 2020;28(3):222–225. doi: 10.4250/jcvi.2019.0091
补充文件