Precision low-dose brachytherapy of prostate cancer under PSMA-receptor molecular visualization
- Authors: Sviridov P.V.1, Rumiantsev P.O.2, Degtyarev M.V.3, Serzhenko S.S.3, Sanin D.B.1,4, Styrov S.V.1, Agibalov D.Y.1, Korenev S.V.5
-
Affiliations:
- Medical center “Doctor Plus”
- Clinics group “My Medical Center”
- Endocrinology Research Centre
- National Medical Research Radiological Center
- I. Kant Baltic Federal University
- Issue: Vol 4, No 3 (2023)
- Pages: 411-426
- Section: Case reports
- Submitted: 18.04.2023
- Accepted: 20.07.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/340815
- DOI: https://doi.org/10.17816/DD340815
- ID: 340815
Cite item
Abstract
Brachytherapy with implantation of micro sources based on isotope 125I is a preferred treatment for localized prostate cancer without signs of germination of the gland capsule and in the absence of signs of metastases (stage cT1-T23aN0M0). Structural imaging methods (ultrasound, computed tomography, and magnetic resonance imaging) do not have high specificity in the differential diagnosis of prostate cancer. Hybrid technologies of radiation imaging (single-photon emission computed tomography/computed tomography, positron emission tomography/computed tomography, and positron emission tomography/magnetic resonance imaging) combine the advantages of high sensitivity of cross-sectional structural imaging methods (computed tomography and magnetic resonance imaging) and high specificity of molecular imaging methods (single-photon emission computed tomography and positron emission tomography) with tumorotropic radiopharmaceuticals. In this original clinical study, based on seven observations of localized prostate cancer (Gleason 6–7), it was shown that the precision of low-dose brachytherapy using 125I micro sources of localized prostate carcinomas, along with targeted biopsy, can be increased using hybrid methods of PSMA-receptor molecular imaging (single-photon emission computed tomography/ computed tomography, positron emission tomography/ computed tomography). The single-photon emission computed tomography/ computed tomography method is more accessible than positron emission tomography/ computed tomography. Moreover, when coupled with cold kits (HYNIC-PSMA), it allows research within any radioisotope diagnostics laboratory equipped with single-photon emission computed tomography/ computed tomography. The innovative technology of PSMA-navigation biopsy and brachytherapy, under the control of hybrid molecular imaging, can be used in primary and recurrent cases of localized prostate cancer, increases the accuracy and reduces the traumatic nature of procedures, and increases the medical and economic efficiency of low-dose brachytherapy with 125I micro sources. Further research is needed to improve the technology and evaluate its long-term results.
Full Text
BACKGROUND
Prostate cancer is the second most common malignant neoplasm in males and the fifth most common cause of death in the world [1]. Screening for prostate cancer based on the level of prostate-specific antigen (PSA) in the blood increases the number of prostate carcinomas detected at an early stage, significantly improving the prognosis of patient survival [2].
One method for treating localized prostate cancer without signs of capsule germination and without metastases (cT1-T2N0M0) is brachytherapy with implantation of 125I-based microsources [3–8].
In brachytherapy, the radiation dose is achieved by spatial distribution of microsources during implantation in the projection of primary prostate carcinoma guided by computed tomography (CT) or ultrasound (US). Implanted microsources are fixed in the tissue, protecting surrounding healthy tissue from irradiation. Simultaneously, safe irradiation borders (2–3 mm) along the periphery of microsources do not lead to side irradiation of risk organs, such as the urethra, bladder, and rectum, which are inevitably affected during prostatectomy, external beam radiation therapy, and high-dose brachytherapy. Some studies of prostate cancer patients at low and moderate risk of tumor recurrence showed better tumor ablation than external beam radiotherapy [4, 5]. The effectiveness of low-dose brachytherapy in patients with localized prostate cancer (pT1-2N0M0) is at least noninferior compared with any other current treatment method, whereas patient tolerability and quality of life are significantly higher [9]. While in the early 2000s, low-dose brachytherapy was combined with external beam radiation therapy for boosting in a moderate risk group, in recent decades, this method has been used more frequently [10].
The brachytherapy method has several advantages, including its low invasiveness (puncture technology), the ability to control the process of installing microsources using structural imaging methods (ultrasound and CT), and the low frequency and low severity of adverse effects [11].
Hybrid radiation imaging technologies, such as single photon emission CT combined with CT (SPECT/CT), positron emission tomography combined with CT (PET/CT), and positron emission tomography combined with magnetic resonance imaging (PET/MRI), combine the high sensitivity of cross-sectional structural imaging methods (CT and MRI) and high specificity of molecular imaging methods (SPECT and PET) with tumor tropic radiopharmaceuticals (RPs).
Prostate-specific membrane antigen (PSMA) in humans is encoded by the FOLH1 gene, which is involved in folate metabolism. It is a zinc-containing transmembrane glycoprotein produced in prostate epithelial cells and known as N-acetyl-L-aspartyl-L-glutamate peptidase or glutamate carboxypeptidase II. Its expression is significantly higher in malignant cells and closely related to tumor aggressiveness, particularly in castration-resistant and metastatic prostate cancer [12].
PSMA-receptor molecular imaging techniques (SPECT and PET) provide additional advantages in determining tumor stage and selecting targets for precision biopsy and brachytherapy of prostate carcinoma [13]. PET/CT with 68Ga-PSMA-11 and 18F-PSMA-1007 and SPECT/CT with 99mTc-HYNIC-PSMA (small-molecule PSMA inhibitors) are accessible in Russian clinical practice. The latter appears highly promising because of its greater availability (significantly more SPECT/CT scanners than PET/CT scanners), easy operation, lower cost, and technological advantages to ensure high precision in biopsy and brachytherapy of localized prostate cancer.
CASE REPORTS
Patients
The study examines treatment outcomes in seven patients aged 65–84 yr (mean: 73 yr; 95% confidence interval [95% CI], 66.8–79.2]) with histologically diagnosed prostate cancer who underwent brachytherapy using 125I-based microsources at the Doctor-Plus clinic in Obninsk, Kaluga region, Russia. All patients had histologically confirmed prostate carcinoma with no signs of regional and/or distant metastases. Five patients had primary tumors. Another two patients had local tumor recurrence in 21 and 28 months after receiving brachytherapy with 125I-based microsources. The tumor stage in two patients was T1N0M0, whereas the stage in four patients was T2N0M0. The stage in one patient was T3aN0M0. In the other case, the patient refused the proposed surgical treatment, which included combined external beam radiation and androgen deprivation therapy.
The Gleason score was 6 (3 + 3) in five cases and 7 (3 + 4) in two cases. Before brachytherapy, blood PSA levels ranged from 2.8 to 12 ng/mL (mean: 8.2 ng/mL).
Methods
Patients with local tumor recurrence were treated with interstitial radiation therapy using 125I-based microsources in the first stage. The time to progression ranged from 12 to 29 months (24.2 [95% CI, 17.8–30.5]).
Clinical examination included standard measurement of serum PSA levels. A venous blood sample was collected in the morning under fasting conditions and prepared in accordance with study protocols. Serum PSA levels were measured using a chemiluminescent immunoassay (sensitivity limits: 0.008–30.0 ng/mL).
Transrectal ultrasound diagnostics was performed using a B/K Medical scanner (USA) to determine the prostate volume and residual urine. Uroflowmetry was performed to assess the maximum volumetric urine flow rate.
In the excretory phase, a pelvic CT with intravenous contrast enhancement (Omnipaque 350 mg/mL, 30 mL) was performed to better visualize the anatomy of organs at risk using a Somatom Scope multidetector (32 slices) CT scanner (Siemens, Germany) with subsequent data transfer for planning interstitial radiation therapy.
A radionuclide study with 99mTc-HYNIC-PSMA was performed using a General Electric Discovery NM/CT 670 hybrid scanner. The finished RP was administered intravenously with a vent visor (intranasal control) at a 6.3 MBq/kg b.w rate. A pelvic area was scanned 2 h after RP administration using a SPECT/CT gamma camera; 60 projections with 30-s exposures per projection, matrix 256 × 256. CT data acquisition parameters were as follows: tube voltage, 120 kV; current (modulated), 80–400 mA; slice thickness, 3.75 mm with 1.25-mm reconstruction; and couch increment, 1 mm. The resulting 3D SPECT/CT image was analyzed using the General Electric Xeleris 4DR data workstation. The identified prostate lesions of focal RP accumulation were indicated on a schematic PI-RADS prostate sector map.
Without anesthesia, a US-guided biopsy was performed transperineally (using SPECT/CT data with 99mTc-HYNIC-PSMA) with a Promag 2.0 biopsy needle and gun. If required, a CT-guided biopsy of the prostate gland and seminal vesicles was performed under spinal anesthesia through pararectal access. Histological biopsy specimens were examined in pathological laboratories with Gleason score determination.
Planning and conducting brachytherapy with 125I sources
Topometry and dosimetry planning were performed for pre-implantation planning using a VariSpeed dosimetry planning system (Varian, USA). That system allowed the DICOM format images of different modalities to be combined.
125I-based microsources were implanted under a CT guide using a Siemens Somatom Scope (Germany) using a 3D stereotactic machine with a hole increment of 2.5 mm to perform targeted brachytherapy. Under spinal anesthesia, the procedure was performed via pararectal access.
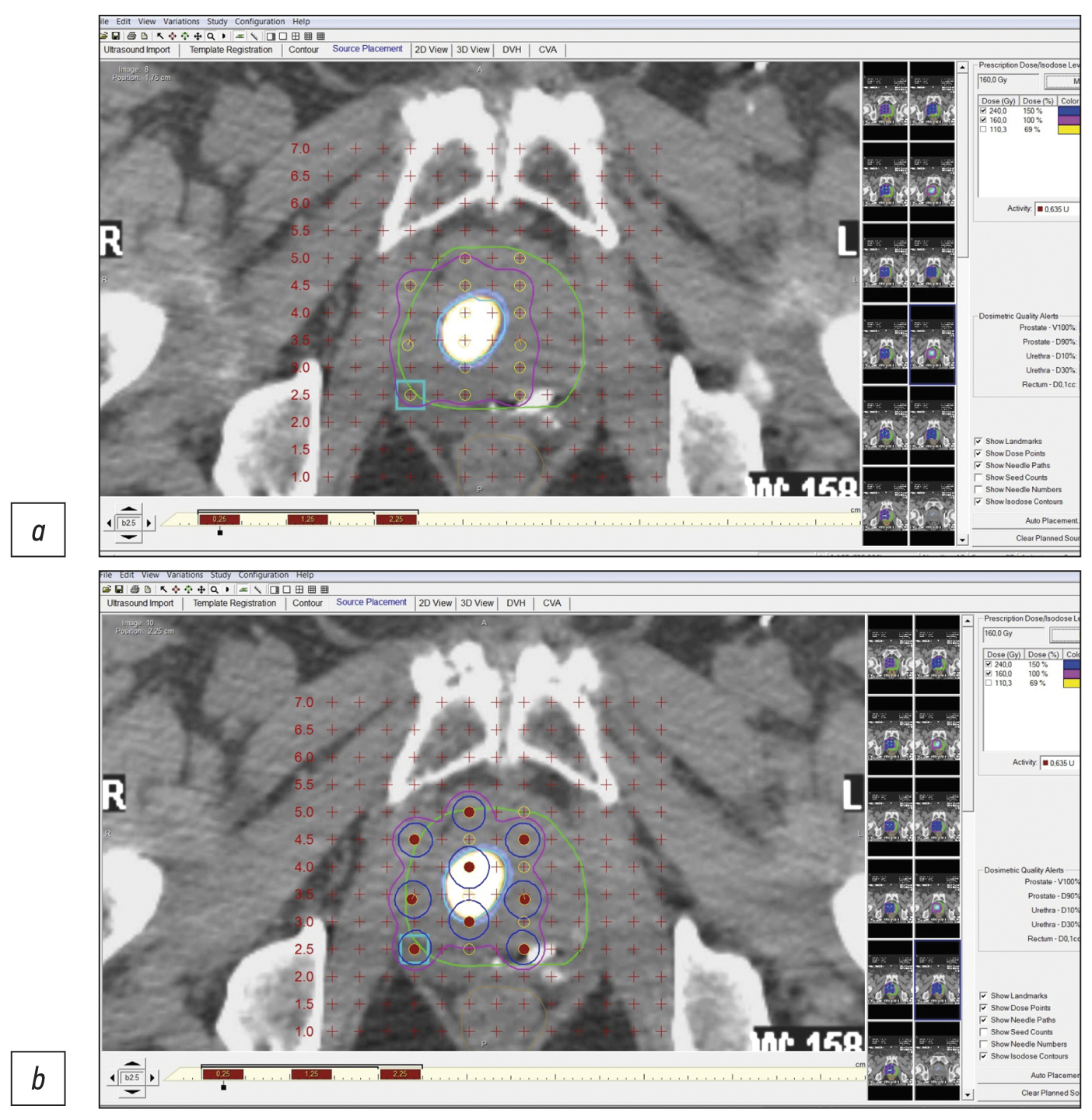
Figure 1 shows an example of pre-implantation brachytherapy planning using 125I sources. Crosses indicate virtual holes in the stereotactic array, which fully corresponds to the array implanted in a patient. Red dots indicate microsources, whereas colored lines indicate isodose distribution.
Fig. 1. Stages of dosimetry planning of brachytherapy localized prostate cancer using 125I microsources considering SPECT/CT with 99mTc-HYNIC-PSMA: (a) topometric marking and (b) topometric marking and dosimetry planning of brachytherapy.
125I microsources in the form of grains (BEBIG LLC, Russia) were used for interstitial implantation. Each source measured 4.5 mm in length and 0.8 mm in diameter. According to the comprehensive examination results and dosimetry planning, the number of sources was ordered individually for each patient (Table 1).
Table 1. Demographic data and clinical and morphological characteristics of patients
Agea, yr | Tumor | Prostate volume/prostate tumor volume, mL | Localization in pancreasd | Planned number of 125I microsources/length of proceduree | Planned number of 125I microsources/length of proceduref | ||
Stage, TNMb | Primary case or relapsec | Gleason score | |||||
Patient 1 84 years old | Т2N0M0 | Primary | 7 (4 + 3) | 40/40 | Right lobe TZa (mid) | 60 75 min | 60 75 min |
Patient 2 79 years old | T2N0M0 | Primary | 6 (3 + 3) | 38/15 | Right lobe TZa (apex), Left lobe TZp (mid) | 60 75 min | 20 25 min |
Patient 3 73 years old | T1N0M0 | Primary | 6 (3 + 3) | 36/14 | Right lobe TZa (apex), Left lobe PZpl (base) PZpl (mid) | 70 87 min | 32 45 min |
Patient 4 68 years old | T3N0M0 | Relapse | 7 (4 + 3) | 38/15 | Right lobe TZp, PZpl (base) | 60 75 min | 20 25 min |
Patient 5 70 years old | T2N0M0 | Relapse | 6 (3 + 3) | 30/15 | Right lobe PZpl (mid) | 70 87 min | 30 40 min |
Patient 6 65 years old | T1N0M0 | Primary | 7 (4 + 3) | 60/15 | Right lobe TZa (mid) | 90 112 min | 30 40 min |
Patient 7 69 years old | T2N0M0 | Primary | 6 (3 + 3) | 78/78 | Right lobe TZa (apex), Left lobe PZpl (base) PZpl (mid) | 32 45 min | 90 112 min |
Note. PSMA, prostate-specific membrane antigen; PZpl (mid/base), posterior zone (middle/basal part); SPECT/CT, single photon emission computed tomography combined with computed tomography; TZa (mid/apex), anterior transition zone (middle/apical part); TZp (mid), posterior transition zone (middle part).
aAge of patients at the time of treatment.
bUICC, 8th ed. (https://oncology.ru/specialist/treatment/tnm/tnm8_summary.pdf).
cMethod of primary treatment.
dInternational standard.
eWithout tumor visualization using SPECT/CT.
fUnder PSMA/SPECT/CT guide.
Brachytherapy requires bowel preparation and antibiotic therapy. Spinal anesthesia with lidocaine/bupivacaine was administered to relieve pain.
The implantation was performed in accordance with 2014 recommendations [12]. During control scans and reconstruction of the resulting images, the placement of 125I microsources was visually monitored.
Patients were discharged 1 day after brachytherapy and were able to return to normal activities within 3–5 days. None of the patients required bladder catheterization or epicystostomy. Mild difficulty urinating was relieved using alpha-blockers in generally accepted doses. There were no reports of early or late radiation effects.
The duration of the brachytherapy procedure depended on the number of implanted 125I microsources and ranged from 25 to 112 min (mean: 51.7 min), which was 34.8% less than the average estimated time (79.4 min) using a standard procedure (without PSMA-SPECT/CT guide).
No severe complications were observed in the early and long-term post-procedure periods (mean: 24.5 months). Mild dysuric symptoms were reported in two patients for several post-procedure days, but they did not require catheterization or drug therapy and resolved spontaneously.
Case report 1
Patient Zh., 84 years old, was admitted with prostate cancer (PSA of 20 ng/mL; adenocarcinoma, Gleason 7 [4 + 3]). On October 9, 2020, CT-guided interstitial radiation therapy with 125I microsources and pararectal access was performed using SPECT/CT data. PSA levels were 0.16 ng/mL 2 yr later, indicating tumor remission. PSA was 0.1 ng/mL on January 14, 2023.
Figure 2 shows SPECT/CT data with 99mTc-HYNIC-PSMA.
Fig. 2. Patient Zh., 8 years old, SPECT/CT with 99mTc-HYNIC-PSMA, axial projection: Site of radiopharmaceutical accumulation in the transition zone of the middle part of the right prostate lobe.
Case report 2
Patient Z., 79 years old, was admitted with diffuse prostatic hyperplasia. PSA level was 4.5 ng/mL. No abnormal lesions were found in the prostate tissue using ultrasound, TRUS, and contrast-enhanced MRI. SPECT/CT with 99mTc-HYNIC-PSMA revealed 15-mm3 sites of RP accumulation in the prostate tissue (Figure 3). Because rectal amputation made US-guided prostate biopsy impossible, a targeted CT-guided biopsy was performed pararectally. A morphological diagnosis was adenocarcinoma, Gleason 6 (3 + 3).
Fig. 3. Patient Z., 7 years old, SPECT/CT with 99mTc-HYNIC-PSMA: Sites of radiopharmaceutical accumulation in transitory zones of both lobes at the border of the middle third and the apex of the prostate gland.
On January 28, 2021, 125I sources were implanted pararectally under a CT guide. In the follow-up examination, PSA levels were 0.2 ng/mL (September 2022) and 0.1 ng/mL (January 14, 2023).
In this case, two problems were revealed: an unknown cause of increased PSA over time and the inability to perform a targeted prostate carcinoma biopsy under US guide. Both problems were successfully resolved using hybrid SPECT/CT with 99mTc-HYNIC-PSMA followed by CT-guided RP accumulation site biopsy, and precision brachytherapy was performed with a smaller number of 125I microsources due to the SPECT/CT pattern.
Case report 3
Patient K., 73 years old, was admitted with diffuse prostatic hyperplasia, urinary retention, an epicystostomy, and a 12.7-ng/mL PSA. A multisite biopsy performed under US guide revealed no evidence of malignant development. SPECT/CT data with 99mTc-HYNIC-PSMA are shown in Figure 4. SPECT/CT revealed a 14-mm3 site of RP accumulation in the prostate tissue. A CT-guided targeted biopsy was performed using pararectal access. A morphological diagnosis was adenocarcinoma, Gleason 6 (3 + 3).
Fig. 4. Patient K., 73 years old, SPECT /CT with 99mTc-HYNIC-PSMA, (a) frontal and (b) axial sections: Sites of radiopharmaceutical accumulation in the anterior part of the transition zone in the apex of the right lobe, posterolateral part of the peripheral zone at the level of the base of the left lobe, and posterolateral part of the peripheral zones at the level of the base and middle third of the left lobe of the prostate gland; physiological accumulation of radiopharmaceuticals in the bladder.
On November 28, 2020, CT-guided focal implantation of 125I sources was performed pararectally. Transurethral resection of the intravesical component was performed, and the epicystostomy was removed. The follow-up examination (November 28, 2022) showed a PSA level of 0.13 ng/mL, spontaneous urination, and 15-cm3 residual urine.
In this case, problems were as follows: inability to identify the cause of elevated PSA, a “large” volume of the prostate gland, and epicystostomy. Considering data on RP accumulation patterns and biopsy results, we were unable to successfully solve these problems and perform successful focal brachytherapy using a hybrid diagnostic use of RP CT-guided biopsy.
Case report 4
Patient F., 68 years old, was admitted with prostate cancer, T3a, which had spread to the prostate gland capsule. In 2013, 125I sources were implanted into the prostate tissue and the extracapsular area. PSA has increased since the end of 2020. SPECT/CT was performed using 99mTc-HYNIC-PSMA due to an increased PSA level of 0.95–2.8 ng/mL after the previous treatment (Figure 5). SPECT/CT revealed a 15-mm3 site of tumor tropic RP accumulation, which was biopsied under the CT guide. A histological diagnosis was prostate adenocarcinoma, Gleason 6 (3 + 3). Dosimetry planning for low-dose brachytherapy was performed using SPECT/CT. On November 03, 2021, 125I microsources were re-implanted according to topometric markings to implement the dosimetry plan. The PSA level was 0.05 ng/mL during the follow-up examination (January 23, 2023).
Fig. 5. Patient F., 68 years old, SPECT/CT with 99mTc-HYNIC-PSMA: Sites of radiopharmaceutical accumulation at the border of the central zone and the posterolateral part of the peripheral zone on the right side at the level of the base of the right prostate lobe. The scan visualizes multiple rods in the prostate gland, implanted during previous brachytherapy.
In this case, SPECT/CT with 99mTc-HYNIC-PSMA allowed for a precision biopsy, which confirmed the local recurrence of prostate cancer and subsequently enabled brachytherapy to be repeated (salvage) under the CT guide.
Case report 5
Patient G., 70 years old, was admitted with prostate cancer (diagnosed in 2005), T2a, adenocarcinoma, Gleason 6 (3 + 3). CT-guided brachytherapy was successfully performed in 2005. The PSA level has increased (1.94–2.31 ng/mL) since 2017. Because of this growth, ultrasound, TRUS, CT, and contrast-enhanced MRI were performed; no data for a local recurrence or another cause of PSA growth were obtained. When the PSA level reached 2.63 ng/mL, SPECT/CT with 99mTc-HYNI-PSMA was performed (Figure 6). Results showed a 15-mm3 site of RP accumulation, and a targeted biopsy of a recurrent prostate carcinoma lesion was performed based on its SPECT/CT location. Adenocarcinoma was confirmed histologically, Gleason 6 (3 + 3). On March 10, 2020, a brachytherapy with reimplantation of 125I microsources was performed. The follow-up examination (December 2022) showed a PSA level of 0.21 ng/mL.
Fig. 6. Patient G., 70 years old, SPECT/CT with 99mTc-HYNIC-PSMA: Site of radiopharmaceutical accumulation in the posterolateral part of the peripheral zone of the middle part of the right prostate lobe. Multiple rods in the prostate gland were implanted during previous brachytherapy.
In this case, SPECT/CT with 99mTc-HYNIC-PSMA enabled prostate carcinoma–targeted biopsy and precision brachytherapy.
Case report 6
Patient M., 65 years old, was diagnosed with benign prostatic hyperplasia. Due to an increased PSA level of 4.5 ng/mL, a US-guided multifocal biopsy was performed with no histological evidence of cancer. A repeat biopsy was performed 3 months later and revealed no malignant tumor. SPECT/CT with 99mTc-HYNIC-PSMA revealed a site of RP accumulation in the prostate tissue (Figure 7). A CT-guided targeted biopsy was performed, and the following diagnosis was morphologically confirmed: adenocarcinoma, Gleason 7 (4 + 3). When planning low-dose brachytherapy using 125I microsources, SPECT/CT data were entered into the dosimetry planning system. On October 4, 2020, the focal implantation of 125I microsources was performed under a CT guide using pararectal access based on data on local isotope accumulation in the prostate tissue. The follow-up examination (January 2023) showed a 0.11-ng/mL PSA level.
Fig. 7. Patient M., 65 years old, SPECT/CT with 99mTc-HYNIC-PSMA: Site of radiopharmaceutical accumulation in the transition zone (at the border of the middle third and the base) of the left prostate lobe.
Case report 7
Patient M., 6 years old, had an increased PSA level of 6.8 ng/mL and underwent a multifocal prostate biopsy at a local clinic. The diagnosis was confirmed morphologically as adenocarcinoma, Gleason 6 (3 + 3), based on data from the transitory zone of the right prostate lobe. Adenocarcinoma elements were not found in other prostate biopsy specimens. Considering MRI evidence of changes in the same zone, implantation with 30 125I sources with a 32-cm3 volume was proposed.
SPECT/CT with 99mTc-HYNIC-PSMA revealed diffuse focal RP accumulation under the base of the bladder. PET/CT with 68Ga-PSMA-11 was additionally performed to more accurately visualize prostate lesions. PET/CT revealed multiple sites of RP accumulation in the prostate gland, indicating that the tumor was multifocal (Figure 8). Implantation planning was adjusted to include the total volume of the prostate gland (78 cm3) using 90 125I microsources (instead of preliminary 30 microsources).
Fig. 8. Patient M., 69 years old, PET/CT with 68Ga-PSMA-11: Sites of radiopharmaceutical accumulation in the prostate gland, multifocal tumor.
On February 25, 2022, implantation was performed for the total volume of the prostate gland. The initial prostate volume for brachytherapy was 32 cm3. PET/CT revealed a volume of 72 cm3 for implantation. The PSA level decreased to 0.31 ng/mL within a year after brachytherapy.
Changes in PSA levels in all patients included are shown in Figure 9.
Fig. 9. Changes in prostate-specific antigen levels in individual patients.
All patients are under clinical supervision and have regular follow-up examinations.
DISCUSSION
A biopsy of prostate lesions is required to confirm the diagnosis and select a management strategy. This procedure is often performed under a US or MRI guide [14-15]. Because radiological structural diagnostics methods (ultrasound, CT, and MRI) do not always visualize lesions of the primary tumor, traumatic multifocal biopsies are often required [15]. False-negative biopsy results are reported in up to 49% of cases. If blood PSA levels increase, a biopsy is repeated [16]. A repeat biopsy is typically performed within 3 months after the initial [16]. Conventional diagnostic methods (ultrasound, CT, and MRI), particularly multiparametric MRI, visualize structural abnormalities but do not differentiate between benign and malignant tumors or assess the borders of a malignant tumor and its multifocal nature [15–17].
The morbidity and false-negative results of conventional prostate biopsy can be reduced with PSMA-receptor imaging guidance using SPECT/CT and/or PET/CT [18–21].
For prostate carcinomas with a low and moderate risk of recurrence (Gleason ≤7), no growth beyond the organ capsule, and evidence of metastases, brachytherapy with 125I microsources is the least traumatic of all recommended treatment options, demonstrating not only high effectivity but also high quality of life [22].
Molecular imaging methods (SPECT and PET) combined with cross-sectional imaging (CT and MRI) differentiates between lesions of malignant prostate tumors with high expression of PSMA receptors and benign structural lesions. As a result, it is possible to perform a US-guided targeted biopsy of positive lesions (considering molecular imaging data) and accurately plan and provide CT-guided brachytherapy.
The paper presents original clinical experience proving the feasibility of precision biopsy and brachytherapy of localized nonmetastatic prostate cancer (pT1-3N0M0) guided by PSMA-receptor molecular imaging (SPECT/CT with 99mTc-HYNIC-PSMA and PET/CT with 68Ga-PSMA-11). Topometric marking and dosimetry planning of brachytherapy of tumor lesions using 125I microsources was also based on the above hybrid molecular imaging data. As a result, topometric imaging of prostate carcinoma lesions enabled us to accurately plan and perform brachytherapy while reducing radiation exposure to healthy tissue and surrounding adjacent organs at risk, hence improving the patient’s quality of life after treatment.
Due to the selective overexpression in prostate cancer tumor cells, PSMA-receptor molecular imaging using positron emission tomography (PET) has been used effectively in clinical practice. If PET/CT with 68Ga-PSMA-11, 18F-DCFPyL (piflufolastat F-18), and 18F-PSMA-1007 is not achievable, SPECT/CT with 99mTc-HYNIC-PSMA may be performed. There are no significant differences in the specificity of PSMA-receptor imaging using PET/CT and SPECT/CT, but the sensitivity of PET/CT is significantly higher; therefore, this method is recommended when results of PSMA-receptor SPECT/CT are negative or questionable [23–25]. However, a valuable advantage of SPECT/CT with 99mTc-HYNIC-PSMA is the ability to improve molecular imaging of tumor lesions in the second phase (delayed, ≥15 h after RP administration) [26].
At the current stage of personalized and precision oncology development, molecular imaging (SPECT and PET), particularly when combined with structural cross-sectional pictures (CT and MRI), enables the detection of tumor lesions based on receptor characteristics or intracellular metabolism. This is especially true for increased precision of external beam radiation therapy, which includes brachytherapy [27].
In our patients, the average implantation time was reduced by one-third (34.8%), proportionally to decreased implanted microsources. Approximately 15–20 min was spent positioning the patient on the tomograph table after spinal anesthesia and installing and positioning the stereotactic attachment to the CT scanner. The length of this process is determined by the patient’s weight and general condition rather than the number of implanted sources. On average, it takes about one and a half minutes to install one needle, position it under CT control, remove styles, load sources, and remove the needles with a control scan. Decreased implantation time is also associated with greater preparedness of the implantation plan due to more accurate visualization of the affected area and its configuration. Therefore, in most cases, there was a significant decrease in the number of microsources, procedure time, personnel radiation exposure, CT scanner occupancy, and rate of injury and subsequent prostate gland swelling (six out of seven). In another case, there were indications for increasing the number of implanted microsources based on PET/CT data using 68Ga-PSMA-11, which was considered when planning and performing the procedure.
Brachytherapy can provide high doses of radiation at tumor sites (average: 160 Gray) and is associated with minimal irradiation of surrounding healthy prostate tissue, including the urethra. It does not irradiate adjacent organs at risk (bladder and rectum).
When SPECT/CT with 99mTc-HYNIC-PSMA and PET/CT with 68Ga-PSMA-11 were used in a clinical population of seven patients with primary (n = 5) and recurrent (n = 2) prostate carcinomas of low and moderate risk, a total number 125I microsources implanted were less (by 36%; 404/282) than the potential number of microsources implanted using standard technique without PSMA-receptor hybrid molecular imaging methods. Simultaneously, as previously reported, molecular imaging reduced the number of sources in six cases while increasing the number of microsources in one case (multifocal tumor). We are not discussing the willingness to “save” microsources; instead, we are talking about the priority of precise diagnostic and treatment processes and personalized increases in the effectiveness and safety of treatment.
A positive biochemical response was achieved in all clinical cases, with PSA levels decreasing to low values and a tendency to decrease further.
ALGORITHM FOR PSMA-PRECISION BRACHYTHERAPY FOR LOCALIZED PROSTATE CANCER
In our cases, the use of hybrid molecular PSMA-receptor imaging (SPECT/CT and PET/CT) methods enabled us to:
- personalize and increase the precision of diagnostic (biopsy) and therapeutic (brachytherapy) procedures in patients with prostate cancer;
- reduce time, increase accuracy, and reduce injury rate associated with morphological verification of the primary tumor: in three of seven cases, a biopsy (previously unsuccessful and traumatic multifocal one) of the tumor was performed using SPECT/CT from the site of accumulation of 99mTc-HYNIC-PSMA, and in all three cases, the presence the tumor was confirmed by histological examination;
- determine the stage of the tumor and exclude regional and distant metastases;
- increase precision of dosimetry and topometric location of 12I microsources to reduce the risk of radiation reactions and improve patient quality of life;
- increase accuracy of microsource implantation due to tumor-targeted planning of interstitial radiation therapy of the tumor lesions with morphological confirmation;
- distribute radioactive sources using a hybrid molecular imaging guide (SPECT/CT, PET/CT, and PET/MRI) and approve the dosimetry plan;
- reduce the waiting time by targeted biopsy of the tumor lesion, considering PSMA-receptor hybrid scintigraphy (SPECT/CT and PET/CT) data on its location; and
- increase the number and topometric plan of placement of 125I microsources during brachytherapy (in this series of cases, the number of microsources was 36% lower, and the procedure time was 34.8% less than the standard treatment plan).
Based on the data given, an algorithm for PSMA-precision brachytherapy for localized prostate cancer was developed and used in the clinical practice of our clinic (Figure 10).
Fig. 10. Algorithm for selecting patients for low-dose brachytherapy, emphasizing increasing precision under PSMA-receptor molecular imaging guide. ku, contrast enhancement; LDR, low-dose rate; PSMA, prostate-specific membrane antigen.
The strength of the study is the use of the original, innovative technology of precision brachytherapy under molecular PSMA-receptor imaging guide using SPECT/CT with 99mTc-HYNIC-PSMA. The half-life of 99mTc is 6 h, allowing for delayed (next day) SPECT/CT of areas of interest and more clearly visualize sites of accumulation of 99mTcwith virtually no background half-lives of 18F and 68Ga are 110 and 68 min, respectively)The limitations of our study are a small clinical sample (seven patients), a short period of follow-up (up to 2 yr), and a lack of MRI disk revision (only conclusions were analyzed, which in all cases were negative regarding tumor lesion localization), as well as the inability to evaluate and compare the diagnostic value of methods. All these aspects should be considered while designing further studies.
We found no reports on the use of such technology in the Russian or foreign literature; therefore, on January 25, 2023, invention patent No. 2788859, “Method of targeted brachytherapy for prostate cancer under hybrid PSMA-receptor scintigraphy guide” was obtained [28].
CONCLUSION
The study suggests that the precision of targeted biopsy and low-dose brachytherapy with 125I microsources for localized prostate carcinomas can be improved using hybrid methods of PSMA-receptor molecular imaging (SPECT/CT and PET/CT). The methods are complementary in terms of diagnostic and therapeutic guides; however, SPECT/CT is more accessible than PET/CT. The availability of cold kits (HYNIC-PSMA) enables one to examine any radioisotope diagnostic laboratory with appropriate equipment.
The innovative technology of PSMA-precision biopsy brachytherapy guided by hybrid molecular imaging can be used for primary and recurrent localized prostate cancer, improving accuracy, reducing invasiveness of procedures, and increasing the medical and economic efficiency of low-dose brachytherapy with 125I microsources.
Further research is required to develop the technology and evaluate long-term treatment outcomes in a larger group of patients.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. P.V. Sviridov — management of patients, brachytherapy procedure conduction, test writing and preparing of images, P.O. Rumiantsev — paper idea, design, coordination, paper writing and edition; M.V. Degtyarev — introduction and interpretation of SPECT/CT and PET/CT, writing of corresponding parts of the paper; S.S. Serzhenko — introduction and interpretation of SPECT/CT, writing of corresponding parts of the paper; D.B. Sanin — dosimetry planning of brachytherapy, writing of corresponding parts of the paper; S.V. Styrov — execution of computed tomography, writing of corresponding parts of the paper; D.Yu. Agibalov — data elaboration, paper edition; S.V. Korenev — assistance in obtaining diagnostic information about treated patients, helping in paper edition.
Consent for publication. Written consent was obtained from all patients for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics Journal.
About the authors
Pavel V. Sviridov
Medical center “Doctor Plus”
Email: p_sviridov73@mail.ru
ORCID iD: 0009-0008-3362-8255
SPIN-code: 4702-3067
Russian Federation, Obninsk
Pavel O. Rumiantsev
Clinics group “My Medical Center”
Author for correspondence.
Email: pavelrum@gmail.com
ORCID iD: 0000-0002-7721-634X
SPIN-code: 7085-7976
Scopus Author ID: 110759
MD, Dr. Sci. (Med.)
Russian Federation, Saint PeterburgMikhail V. Degtyarev
Endocrinology Research Centre
Email: germed@mail.ru
ORCID iD: 0000-0001-5652-2607
SPIN-code: 7725-7831
Russian Federation, Moscow
Sergey S. Serzhenko
Endocrinology Research Centre
Email: vv1ld@yandex.ru
ORCID iD: 0000-0003-2326-1396
SPIN-code: 4713-8986
Russian Federation, Moscow
Dmitry B. Sanin
Medical center “Doctor Plus”; National Medical Research Radiological Center
Email: dimitresko82@yandex.ru
ORCID iD: 0009-0004-2047-4921
SPIN-code: 8939-9101
Cand. Sci. (Biol.)
Russian Federation, Obninsk; ObninskSergey V. Styrov
Medical center “Doctor Plus”
Email: rizost@yandex.ru
ORCID iD: 0000-0003-4315-8855
SPIN-code: 9019-8520
Scopus Author ID: 924845
Russian Federation, Obninsk
Dmitry Yu. Agibalov
Medical center “Doctor Plus”
Email: agibalovd@bk.ru
ORCID iD: 0000-0003-2995-7140
SPIN-code: 6938-5804
Russian Federation, Obninsk
Sergey V. Korenev
I. Kant Baltic Federal University
Email: korenevsv@mail.ru
ORCID iD: 0000-0003-2310-0576
SPIN-code: 5257-4476
MD, Dr. Sci. (Med.), Professor
Russian Federation, KaliningradReferences
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Nyame YA, Gulati R, Tsodikov A, et al. Prostate-Specific antigen screening and recent increases in advanced prostate cancer. JNCI Cancer Spectr. 2021;5(1):pkaa098. doi: 10.1093/jncics/pkaa098
- Pommier P, Ferré M, Blanchard P, et al. Prostate cancer brachytherapy: SFRO guidelines 2021. Cancer Radiotherap. 2022;26(1-2):344–355. doi: 10.1016/j.canrad.2021.11.019
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi: 10.1016/j.annonc.2020.06.011
- Mottet N, van der Berg R, Briers E, et al. EAU-eanm-estro-esur-siog guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042
- Nosov DA, Volkova MI, Gladkov OA, et al. Practical recommendations for the treatment of prostate cancer. Malignant Tumors. Practical recommendations RUSSCO. 2022;12(#3s2):607–626. (In Russ). doi: 10.18027/2224-5057-2022-12-3s2-607-626
- Tsumura H, Tanaka N, Oguchi T, et al. Comparative effectiveness of low-dose-rate brachytherapy with or without external beam radiotherapy in favorable and unfavorable intermediate-risk prostate cancer. Sci Rep. 2022;12(1):11023. doi: 10.1038/s41598-022-15028-6
- Tanaka N, Asakawa I, Hasegawa M, Fujimoto K. Low-dose-rate brachytherapy for prostate cancer: A 15-year experience in Japan. Int J Urol. 2020;27(1):17–23. doi: 10.1111/iju.14098
- Fellin G, Mirri MA, Santoro L, et al. Low dose rate brachytherapy (LDR-BT) as monotherapy for early stage prostate cancer in Italy: Practice and outcome analysis in a series of 2237 patients from 11 institutions. Br J Radiol. 2016;89(1065):20150981. doi: 10.1259/bjr.20150981
- Okamoto K, Okuyama K, Kohno N, Tsugawa T. Clinical outcomes of low-dose-rate brachytherapy based radiotherapy for intermediate risk prostate cancer. J Contemp Brachytherapy. 2020;12(1):6–11. doi: 10.5114/jcb.2020.92405
- Cunha JA, Flynn R, Bélanger C, et al. Brachytherapy future directions. Semin Radiat Oncol. 2020;30(1):94–106. doi: 10.1016/j.semradonc.2019.09.001
- Afshar-Oromieh A. PSMA-ligand imaging in the diagnosis of prostate cancer. In: Clinical Nuclear Medicine: Second Edition. Springer International Publishing; 2020. Р. 755–763. doi: 10.1007/978-3-030-39457-8_25
- Zippel C, Ronski SC, Bohnet-Joschko S, et al. Current status of PSMA-radiotracers for prostate cancer: Data analysis of prospective trials listed on clinicaltrials.gov. Pharmaceuticals. 2020;13(1):12. doi: 10.3390/ph13010012
- Zyryanov AV, Oshchepkov VN, Sviridov PV, et al. Recommendations for the treatment of prostate cancer with low-dose permanent interstitial radiation therapy (brachytherapy). Expert meeting of the Association of Brachytherapists of Russia (OBR), October 4, 2014, Moscow. Experimental Clin Urol. 2015;(2):37–46. (In Russ).
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777 doi: 10.1056/nejmoa1801993
- Sazuka T, Imamoto T, Namekawa T, et al. Analysis of preoperative detection for apex prostate cancer by transrectal biopsy. Prostate Cancer. 2013;2013:705865. doi: 10.1155/2013/705865
- Tewes S, Peters I, Tiemeyer A, et al. Evaluation of MRI/ Ultrasound fusion-guided prostate biopsy using transrectal and transperineal approaches. Biomed Res Int. 2017;2017:2176471. doi: 10.1155/2017/2176471
- Qiu DX, Li J, Zhang JW, et al. Dual-tracer PET/CT-targeted, mpMRI-targeted, systematic biopsy, and combined biopsy for the diagnosis of prostate cancer: A pilot study. Eur J Nucl Med Mol Imaging. 2022;49(8):2821–2832. doi: 10.1007/s00259-021-05636-1
- Donato P, Morton A, Yaxley J, et al. 68Ga-PSMA PET/CT better characterizes localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging. 2020;47(8):1843–1851. doi: 10.1007/s00259-019-04620-0
- Zhang LL, Li WC, Xu Z, et al. 68Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur J Nucl Med Mol Imaging. 2021;48(2):483–492. doi: 10.1007/s00259-020-04863-2
- Duan H, Ghanouni P, Daniel B, et al. A pilot study of 68Ga-PSMA11 and 68Ga-RM2 PET/MRI for biopsy guidance in patients with suspected prostate cancer. J Nuclear Med. 2022;64(5):744–750. doi: 10.2967/jnumed.122.264448
- Chin J, Rumble RB, Kollmeier M, et al. Brachytherapy for patients with prostate cancer: American Society of Clinical Oncology / Cancer Care Ontario joint guideline update. J Clin Oncol. 2017;35(15):1737–1745. doi: 10.1200/JCO.2016.72.0466
- Basu S, Alavi A. SPECT-CT and PET-CT in oncology: An overview. Curr Med Imaging Rev. 2011;7(3):202–209. doi: 10.2174/157340511796411168
- Soldatov A, von Klot CA, Walacides D, et al. Patterns of progression after 68Ga-PSMA-Ligand PET/CT-Guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2019;103(1):95–104. doi: 10.1016/j.ijrobp.2018.08.066
- Werner P, Neumann C, Eiber M, et al. [99cmTc]Tc-PSMA-I&S-SPECT/CT: experience in prostate cancer imaging in an outpatient center. EJNMMI Res. 2020;10(1):45. doi: 10.1186/s13550-020-00635-z
- Berliner C, Steinhelfer L, Chantadisai M, et al. Delayed imaging improves lesion detectability in [99mTc]Tc-PSMA-I&S SPECT/CT in recurrentprostate cancer. J Nucl Med. 2023;64(7):1036–1042. doi: 10.2967/jnumed.122.265252
- Rumyantsev PO. The increasing role of functional imaging methods for navigation of remote radiotherapy and brachytherapy on the example of prostate cancer. Digital Diagnostics. 2022;2(4):488–497. (In Russ). doi: 10.17816/DD96197
- Patent RUS № RU 2788859 С2. Agibalov DYu, Degtyarev MV, Rumyantsev PO, et al. Method of targeted brachytherapy of prostate cancer under the navigation of hybrid PSMA-receptor scintigraphy. Available from: https://yandex.ru/patents/doc/RU2788859C2_20230125. Accessed: 15.08.2023.
Supplementary files