Frequency of various cardiac complications in children with repaired tetralogy of Fallot identified by computer tomography
- Authors: Kabdullina A.M.1, Sinitsyn V.E.2, Rakhimzhanova R.I.1, Dautov T.B.3, Saduakassova A.B.4, Kaliyev B.B.1, Bastarbekova L.A.1, Moldakhanova Z.A.1
-
Affiliations:
- Astana Medical University
- Lomonosov Moscow State University
- Department of the Radiology of National Research Cardiac Surgery Center
- Medical Centre Hospital of President’s Affairs Administration of the Republic of Kazakhstan
- Issue: Vol 4, No 3 (2023)
- Pages: 268-279
- Section: Original Study Articles
- Submitted: 02.05.2023
- Accepted: 09.06.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/375285
- DOI: https://doi.org/10.17816/DD375285
- ID: 375285
Cite item
Abstract
BACKGROUND: Tetralogy of Fallot represents 7–10% of all cases of congenital heart disease, as it occurs in approximately 0.5 per 1,000 live births and is the second most common form of complex congenital heart disease. Advances in diagnosis, surgical techniques, and postoperative treatment have led to an increasing number of patients reaching adulthood, with a dramatic increase in the survival rate to almost 90% at 30 years, thereby creating a need for long-term monitoring of certain anatomic parameters to identify complications in a timely manner. This study aimed to investigate the frequency of computed tomography detected complications after radical correction of Tetralogy of Fallot in pediatric patients.
AIM: to identify markers between the most frequency computed tomography detected complications after repair of Tetralogy of Fallot in pediatric patients.
MATERIALS AND METHODS: A retrospective analysis was conducted on 613 patients with Tetralogy of Fallot from October 2011 to June 2020. The study included a total of 116 patients (69 men and 47 women) who experienced complications after a repair of Tetralogy of Fallot, as identified by computed tomography. At the time of repair of Tetralogy of Fallot, the patient’s average age ranged from 10 to 36 months (mean: 12 months), average body weight was 21 kg, average height was 105.4 cm, and average body surface area was 0.74 m2. The patients’ median age at the time of the computed tomography examination was 17.5 years (age range: 7–36 years).
RESULTS: Among the 116 patients who exhibited complications after an repair of Tetralogy of Fallot, 49 had a pulmonary artery stenosis, 92 had a pulmonary artery branch stenosis (56 of them of the left main pulmonary artery branch, and 36 of them of the right main pulmonary artery branch), 8 had a right ventricular outflow tract stenosis, 32 had a ventricular septal defect, 1 had a shunt thrombosis, 12 had a postoperative deformation of the pulmonary artery, 10 exhibited a marked right ventricular dilatation, 2 had an right ventricular outflow tract aneurysm, and 6 suffered from conduit calcification and stenosis. Moreover, patients with left main pulmonary artery branch stenosis had a 6.5 times greater chance of developing an right main pulmonary artery branch stenosis in (p <0.001).
CONCLUSION: The most frequently computed tomography detected complications after a repair of Tetralogy of Fallot were pulmonary artery stenosis and pulmonary artery branch stenosis. Patients with pulmonary artery stenosis and pulmonary artery branch stenosis exhibit no significant differences in terms of age, anthropometric parameters (height, weight, and body surface area), and gender distribution in the presence or absence of different stenosis types (pulmonary artery, right main pulmonary artery branch, or left main pulmonary artery branch). However, an right main pulmonary artery branch stenosis increases the chances of developing an left main pulmonary artery branch stenosis.
Full Text
INTRODUCTION
Tetralogy of Fallot (ToF) represents 7%–10% of all congenital heart disease (CHD) cases, as it occurs in 0.5/1,000 live births and is the second most common form of complex CHD (1). Advances in diagnosis, surgical techniques, and postoperative treatment have led to an increasing number of patients reaching adulthood, with a dramatic increase in the survival rate to almost 90% at 30 years (2), thereby creating a need for long-term monitoring of certain anatomic parameters so that the complications can be identified on time.
After repair of ToF (rToF), imaging tools should be used to assess the right ventricular (RV) volume and any pressure overload due to the tricuspid and pulmonary regurgitation or stenosis, and scan for any RV and left ventricular systolic and diastolic dysfunction, the presence of any postoperative scars, the presence of any RV aneurysms and fibrosis, as well as the presence of any associated anomalies, such as aortic root dilation and aortic insufficiency (1).
Noninvasive imaging plays a critical role in the follow-up of patients after a rToF. Transthoracic echocardiography (TTE) is the primary and routine clinical investigation tool for anatomical and the functional assessment required in these cases. However, it is important to note that the results of the TTE are largely dependent on the operators. Cardiac computed tomography (CT) and magnetic resonance imaging (MRI) have been generally regarded as the complementary tools for this purpose (3)(4).
Multidetector computed tomography (MDCT), with its high spatial, and temporal resolution, plays a crucial role in evaluating complex anatomical findings in both unrepaired and repaired ToF patients (2). A cardiac CT can provide the necessary functional and anatomical information for making informed decision-making in complex CHD cases. Image interpretation is aided by the knowledge of the common approaches to operative repair and the residual hemodynamic abnormalities (5). Technical advances have allowed high-quality images and a marked decrease in the radiation dose of the cardiac CT. For selected indications, cardiac CT may provide better information with a lower risk compared with other diagnostic modalities, proving helpful in the ToF evaluation (1). In contrast, cardiac MRI in small children is mainly limited due to the long examination time requiring conscious sedation or general anesthesia, and the relatively low spatial resolution, which may partly explain the sparse cardiac MRI data in young children with ToF in the literature. Moreover, CT imaging does not interfere with pacemakers and defibrillators, even with the older models that are noncompatible with cardiac magnetic resonance models. The aim of this study was to identify markers between the most frequency CT-detected complications after an rToF in pediatric patients.
MATERIALS AND METHODS
Ethical considerations
The study was approved by the Institutional Review Ethics Committee of the National Research Cardiac Surgery Center (approval #01-92/2021 on 22 April 2021). No anticipated risks to the participants were identified. During data collection, all personal information of the patients was encoded and depersonalized to safeguard patient rights and prevent the disclosure of personal information. Researchers received the electronic database that was limited to the information regarding the demographic and clinical characteristics of the patients, which was analyzed, and reported in an aggregated form only, thereby assuring its confidentiality. This study was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients or their legal guardians prior to participation.
Study venue and patients
This is a retrospective study, conducted at a tertiary, highly specialized hospital. CT examinations were performed from December 2011 to June 2020 on patients with a history of rToF who were referred for a cardiac CT examination as part of a clinically necessary standard of care, by the Cardiothoracic Surgery, and the Cardiology Departments. A retrospective analysis of 613 patients with ToF was carried out. Written informed consent was obtained from each participant and the parents of minors before their data was included in the study. We identified 116 patients (69 men, 47 women) with complications of the rToF through their CT results. The patients’ average age when the rToF was performed ranged from 10 to 36 months (mean: 12 months), and the patients had an average body weight of 21 kg, an average height of 105.4 cm, and an average body surface area (BSA) of 0.74 m2. The patients’ median age at the time of the CT examination was 17.5 years (age range: 7–36 years) (Table 1).
Table 1. General characteristics of participants (N = 116)
Patient characteristics | Frequency/mean |
Gender, n (%) | |
Male | 69 (59) |
Female | 47 (41) |
Age at initial correction (months) | 12 (10–36) |
Previous shunt procedure, n (%) | 25 (21,5) |
Initial correction, n (%) | |
Transannular patch, n (%) | 67 (57,7) |
Myectomy/valvulotomy, n (%) | 40 (35,5) |
Contegra valved conduit, n (%) | 9 (7,7) |
Weight, kg | 21,08 |
Height, cm | 105,4 |
Body surface area, m2 | 0,7 |
Note: cm, centimeter; kg, kilogram; m2, meter square.
Patients with the following conditions were excluded from the study population: (i) iodine allergy, (ii) high creatinine levels, and (iii) adults with unrepaired ToF (n = 4). Subsequently, the following inclusion criteria were applied: (i) presence of an informed consent and (ii) prior correction of ToF.
In a total of 613 cardiac CT examinations screened, 138 were performed before any surgical procedures, 20 were performed after a palliative operation (such as shunt, RV outflow tract (RVOT) stent placement, or RVOT widening), 285 were performed after a total surgical rToF (consisting of a closure of the ventricular septal defect (VSD) and of a relief of the RVOT obstruction), and 26 were performed after pulmonary valve replacement (that comprised four treatment stages).
Cardiac CT
Children who had undergone palliative operations and had rTOF underwent scanning using a SOMATOM Definition AS 64 CT scanner at the Radiology Department. The scans were conducted with prospective cardiosynchronization and reconstruction, utilizing a slice thickness of 0.6 mm.
All patients were examined in a supine position, head first, with an intravenous bolus administration through an automatic tubeless CT-injector Ohiotandem, and an infusion rate of 1–2 mL/sec.
Infants and young children up to 4 years of age were sedated using oral chloral hydrate (75 mg/kg of body weight) and ketamine (1 mg/kg of body weight), while older children, and adults underwent the examination without sedation.
To optimize the radiation dose during CT, our institution employed standard body-size-adapted protocols. These protocols were based on combinations of body weight and the size of the cardiac shadow observed on scout images, which allowed us to determine the optimal tube current–time product per rotation (6).
Data analysis and interpretation
Descriptive data are presented as percentages (for categorical variables) and as mean ± standard deviation or median (interquartile range), as appropriate. The cohorts were divided into two groups: survivors and deceased patients. Categorical variables were compared using χ2 tests, while continuous variables were compared using t-tests or Mann–Whitney U tests.
Comparisons among the three groups were made using a bivariate analysis for normally distributed data. The same analysis was used to assess the association between PA stenosis and other variables. The Pearson’s correlation coefficient or the Spearman’s rank coefficient was used to assess the correlation between the functional parameters examined in the three different groups. P-values were two-sided and were reported as significant at a p < 0.005 in all analyses. All statistical analyses were performed using the SPSS software (version 24.0; IBM Corp.).
RESULT
This retrospective study was carried out from October 2011 to June 2020, and 613 patients with ToF were involved. We identified 116 patients with complications after a total rToF through their CT examinations.
In patients post-rToF, 49 had PA stenosis (Table 2), and 92 demonstrated a PA branch stenosis. In 56 of them, the stenosis affected the left main PA branch (Table 3), while in 36 of them; the stenosis affected the right main PA branch (Table 4). Moreover, 8 patients were diagnosed with RVOT stenosis, 32 had developed a VSD, 1 patient had a shunt thrombosis, 12 suffered from a postoperative deformation of the PA, 10 exhibited a marked RV dilatation, 2 had an RVOT aneurysm, and 6 suffered from conduit calcification and stenosis (Figure 1).
Table 2. Comparison of medical characteristics between patients with and without PA stenosis (N = 116)
Variable | No (n=67) | Yes (n=49) | p-value |
Age, months | 28,5 (29,5) | 24,6 (27) | 0,48 |
Height, cm | 107,1 (29,4) | 103,1 (29,8) | 0,47 |
Weight, kg | 22,6 (17,4) | 19 (12,9) | 0,23 |
BSA | 0,75 (0,29) | 0,72 (0,34) | 0,5 |
Gender | 0,14 | ||
Male | 36 (52,2%) | 33 (47,8%) | |
Female | 31 (66%) | 16 (34%) | |
Palliative operation | 0,8 | ||
No | 52 (57,1%) | 39 (42,9%) | |
Yes | 15 (60%) | 10 (40%) | |
ToF type | 0,08 | ||
Pulmonary artery stenosis | 56 (55%) | 46 (45%) | |
Pulmonary atresia | 11 (79%) | 3 (21%) | |
Operation type | 0,69 | ||
TAP | 38 (56,7%) | 29 (43,3%) | |
No TAP | 23 (56,1%) | 18 (43,9%) | |
Conduit | 6 (75%) | 2 (25%) | |
Shunt thrombosis | 0,58 | ||
No | 66 (57,4%) | 49 (42,6%) | |
Yes | 1 (100%) | 0 | |
VSD | 0,84 | ||
No | 49 (58,3%) | 35 (41,7%) | |
Yes | 18 (56,3%) | 14 (43,7%) | |
RVOT stenosis | 0,26 | ||
No | 61 (56,5%) | 47 (43,5%) | |
Yes | 6 (75%) | 2 (25%) | |
RVOT aneurysm | 0,33 | ||
No | 65 (57%) | 49 (43%) | |
Yes | 2 (100%) | 0 | |
PA deformation | 0,61 | ||
No | 60 (58%) | 44 (42,3%) | |
Yes | 7 (58,3%) | 5 (41,7%) | |
RPA stenosis | 0,93 | ||
No | 46 (57,5%) | 34 (42,5%) | |
Yes | 21 (58,3%) | 15 (41,7%) | |
LPA stenosis | 0,03* | ||
No | 29 (48,3%) | 31 (51,7%) | |
Yes | 38 (67,9%) | 18 (32,1%) | |
RV dilation | 0,58 | ||
No | 61 (57,6%) | 45 (42,4%) | |
Yes | 6 (60%) | 4 (40%) | |
Conduit calcification and stenosis | 0,19 | ||
No | 62 (56,4%) | 48 (43,6%) | |
Yes | 5 (83,3%) | 1 (16,7%) | |
Note: *Chi-square test. ORLPAstenosis yes = 0.44. Interpretation: The odds ratio of PA stenosis for those who have LPA stenosis is 0.44 times (56% lower) than for those who does to have LPA stenosis.
Table 3. Comparison of medical characteristics between patients with and without LPA stenosis (N = 116)
Variable | No (n=60) | Yes (n=56) | p-value |
Age, months | 29,3 (32,2) | 24,3 (24,1%) | 0,35 |
Height, cm | 108 (28,3) | 102 (30,7) | 0,3 |
Weight, kg | 20,3 (12,4) | 21,9 (18,7) | 0,6 |
BSA | 0,76 (0,33) | 0,72 (0,29) | 0,46 |
Gender | 0,1 | ||
Male | 40 (58%) | 29 (42%) | |
Female | 20 (42,6%) | 27 (57,4%) | |
Palliative operation | 0,35 | ||
No | 45 (49,5%) | 46 (50,5%) | |
Yes | 15 (60%) | 10 (40%) | |
ToF type | 0,06 | ||
Pulmonary artery stenosis | 56 (55%) | 46 (45%) | |
Pulmonary atresia | 4 (28,6%) | 10 (71,4%) | |
Operation type | 0,96 | ||
TAP | 34 (50,8%) | 33 (49,2%) | |
No TAP | 22 (53,7%) | 19 (46,3%) | |
Conduit | 4 (50%) | 4 (50%) | |
Shunt thrombosis | 0,52 | ||
No | 59 (51,3%) | 56 (48,7%) | |
Yes | 1 (100%) | 0 | |
VSD | 0,024* | ||
No | 38 (45,2%) | 46 (54,8%) | |
Yes | 22 (68,8%) | 10 (31,3) | |
RVOT stenosis | 0,6 | ||
No | 56 (51,8%) | 52 (48,2%) | |
Yes | 4 (50%) | 4 (50%) | |
RVOT aneurysm | 0,23 | ||
No | 60 (52,6%) | 54 (47,4%) | |
Yes | 0 | 2 (100%) | |
PA deformation | - | ||
No | 56 (53,8%) | 48 (46,2%) | 0,15 |
Yes | 4 (33,3%) | 8 (66,7%) | |
RV dilation | 0,42 | ||
No | 54 (51%) | 52 (49%) | |
Yes | 6 (60%) | 4 (40%) | |
Conduit calcification and stenosis | 0,63 | ||
No | 57 (51,8%) | 53 (48,2%) | |
Yes | 3 (50%) | 3 (50%) | |
Note: *Chi-square test. ORVSD yes = 0.38. Interpretation: The odds of developing LPA stenosis is 0.38 times (62% lower) for those with VSD tan for those without VSD.
Table 4. Comparison of medical characteristics between patients with and without RPA stenosis (N = 116)
Variable | No (n=80) | Yes (n=36) | p-value |
Age, months | 26 (29,5) | 28,7 (26,8) | 0,64 |
Height, cm | 107,8 (28,2) | 100 (31,9) | 0,19 |
Weight, kg | 20 (12) | 23,6 (22) | 0,26 |
BSA | 0,75 (0,32) | 0,72 (0,29) | 0,61 |
Gender | 0,32 | ||
Male | 50 (72,5%) | 19 (27,5%) | |
Female | 30 (63,8%) | 17 (36,2%) | |
Palliative operation | 0,35 | ||
No | 64 (70,3%) | 27 (29,7%) | |
Yes | 16 (64%) | 9 (36%) | |
ToF type | 0,45 | ||
Pulmonary artery stenosis | 71 (69,6%) | 31 (30,4%) | |
Pulmonary atresia | 9 (64,3%) | 5 (35,7%) | |
Operation type | 0,86 | ||
TAP | 47 (70,2%) | 20 (29,8%) | |
No TAP | 28 (68,3%) | 13 (31,7%) | |
Conduit | 5 (62,5%) | 3 (37,5%) | |
Shunt thrombosis | 0,69 | ||
No | 79 (68,7%) | 36 (31,3%) | |
Yes | 1 (100%) | 0 | |
VSD | 0,68 | ||
No | 57 (67,9%) | 27 (32,1%) | |
Yes | 23 (71,9%) | 9 (28,1%) | |
RVOT stenosis | 0,52 | ||
No | 74 (68,5%) | 34 (31,5%) | |
Yes | 6 (75%) | 2 (25%) | |
RVOT aneurysm | 0,09 | ||
No | 80 (70,2%) | 34 (29,8%) | |
Yes | 0 | 2 (100%) | |
PA deformation | 0,55 | ||
No | 72 (69,2%) | 32 (30,8%) | |
Yes | 8 (66,7%) | 4 (33,3%) | |
LPA stenosis | <0,001* | ||
No | 52 (86,7%) | 8 (13,3%) | |
Yes | 28 (50%) | 28 (50%) | |
RV dilation | 0,62 | ||
No | 73 (68,9%) | 33 (31,1%) | |
Yes | 7 (70%) | 3 (30%) | |
Conduit calcification and stenosis | 0,39 | ||
No | 75 (68,2%) | 35 (31,8%) | |
Yes | 5 (83,3%) | 1 (16,7%) | |
Note: *Chi-square test. ORLPA yes = 6.5. Interpretation: The odds of developing RPA stenosis is 6.5 times (550%) higher for those with LPA stenosis than for those without LPA stenosis.
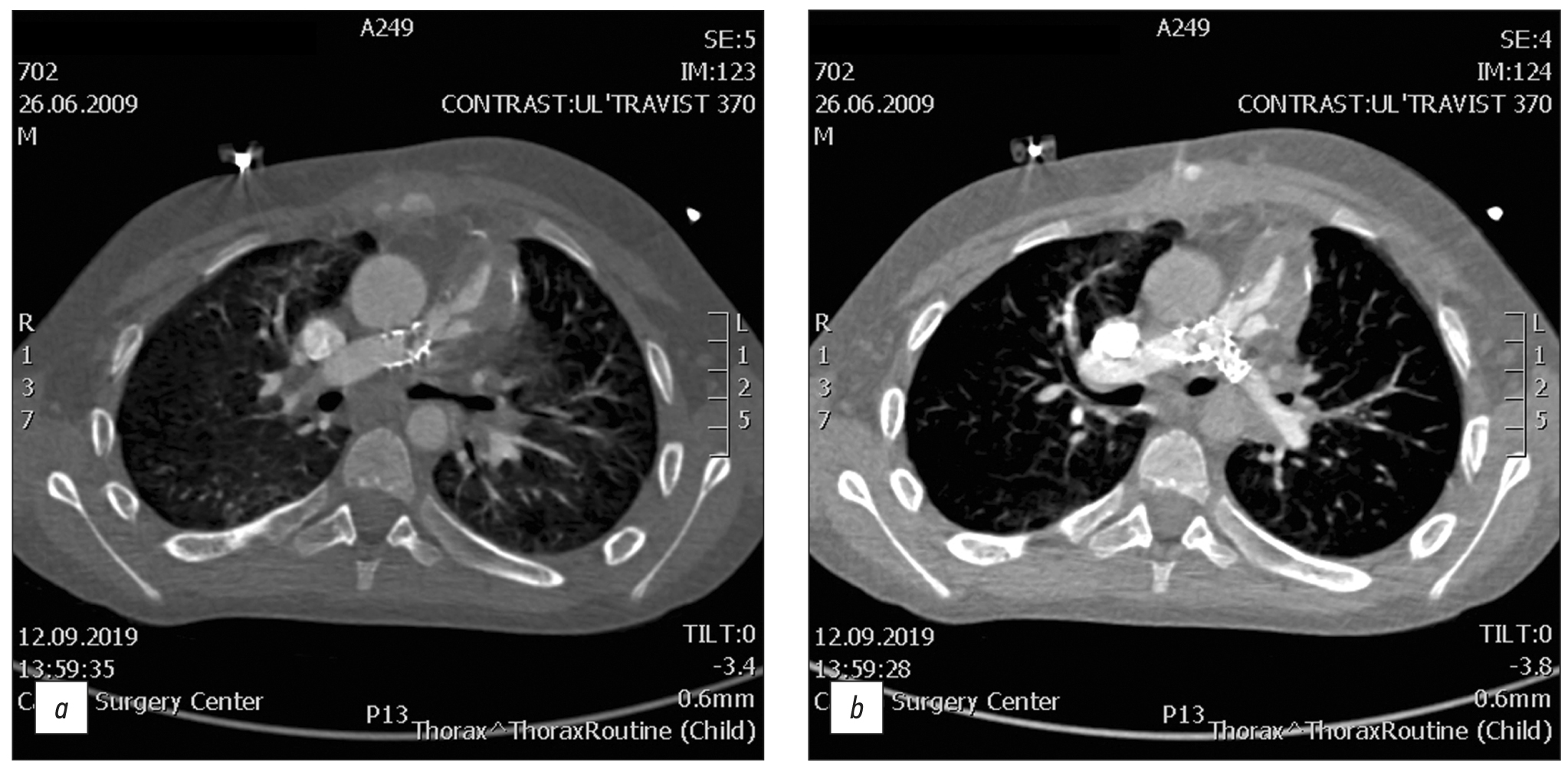
Fig. 1. CT of valve-containing conduit Contegra No. 16 and bilateral stents Palmaz Genesis XD 19-10.
A 12-year-old male with repaired tetralogy of Fallot, after implantation of valve-containing conduit Contegra No. 16 and bilateral stents Palmaz Genesis XD 19-10. Cardiac CT image clearly demonstrates thrombosis conduit. All the complications detected by CT were validated with angiography and were operated on.
The most frequent complications observed were PA stenosis and PA branch stenosis. We decided to compare the medical characteristics between the patients with and without PA and PA branch stenosis by conducting a bivariate analysis and by calculating the odds ratios.
Among the three comparison pairs examined, no significant differences were found in terms of patients’ age, anthropometric parameters (height, weight, and BSA), and gender distribution in the presence or absence of the different stenosis types (PA, RPA, and LPA). Moreover, there were no significant differences regarding the type of ToF, presence of shunt thrombosis and palliative surgery, or the type of surgery performed (p > 0.05). In fact, the data of the comparison groups were comparable according to the aforementioned criteria.
Patients with a VSD exhibited a lower risk for developing LPA stenosis (odds ratio or: 0.039; 95% confidence interval or 95% CI: 0.160.89; p < 0.005). At the same time, the presence of VSD was not associated with the risk of developing a stenosis of the PA or of the RPA (p > 0.005).
As indicated in Tables 2 and 3, there were no significant associations between the presence of an RVOT stenosis or aneurysm and the development of PA, RPA, or LPA stenosis. Similar results were obtained for the presence or absence of a PA deformation (p > 0.005).
The development of PA stenosis was significantly lower (OR: 0.44; 95% CI 0.21–0.94; p < 0.005) among patients with an LPA stenosis, but not with an RPA stenosis (p > 0.005). At the same time, patients with an LPA stenosis had a 6.5 times (95% CI: 2.62–16.15; p < 0.001) greater chance of developing an RPA stenosis.
No significant associations were found between the RV dilation or the conduit calcification and stenosis and the development of a PA, an RPA, or an LPA stenosis (p > 0.005).
DISCUSSION
An increasing number of adult patients with CHD continue to require life-long diagnostic imaging surveillance through cardiac CT and MRI. These patients are characterized by a large spectrum of unique anatomical and functional changes resulting from either single- or multistage palliation and surgical correction. Radiologists involved in the diagnostic task of monitoring treatment effects and detecting potential complications should be familiar with common cardiac CT and MRI findings observed in patients with repaired, complex adult CHD (7).
Due to its high spatial and temporal resolution and the capability of providing high-quality three-dimensional reconstructed images, MDCT has become the primary modality for several patients, predominantly for the evaluation of PA and of the major aortopulmonary collateral arteries (8). In this study, we found that the most frequent complications occurring after a total rToF are PA and PA branch stenosis.
Echocardiography is still considered as the first modality in the evaluation of the postoperative complications following CHD treatment procedures, due to its known advantages of being safe, affordable, and lacking ionizing radiation, along with its superior capabilities in the delineation of the intracardiac anatomy and of the cardiac physiological functions. However, due to its dependency on its operator and the limitation of its narrow acoustic window, echocardiography faces difficulties in the visualization of extracardiac anatomy as well as a number of challenges in the quantitative assessment of the RV size, function, and valve regurgitation (9).
Cardiac CT and cardiac MRI are considered as minimally invasive techniques in the evaluation of the extracardiac postoperative vascular complications. However, in contrast to cardiac MRI, CT is superior in its inherent capability to identify intracardiac anatomical elements, to assess small vessel anatomy (including pulmonary veins, distal PA branches, and aortopulmonary collaterals), and to identify functional, and structural abnormalities or postoperative complications following CHD surgical procedures. Therefore, CT is steadily becoming an invaluable imaging modality capable of filling the gap between echocardiography, cardiac catheterization, and cardiac MRI (10).
Different types of conduits are used in the surgical rToF. The immediate postoperative results are excellent, but with time, progressive conduit obstruction occurs due to patient-prosthesis mismatch, distal anastomotic stenosis, conduit kinking, thrombosis, and the development of calcifications. MDCT can accurately assess the exact mechanism of such conduit obstructions, as well as assess the stenosis level, degree, and extension (2).
Transannular patch repair frequently leads to RVOT dilatation and aneurysms, chronic and severe pulmonary regurgitation, and subsequent RV dilatation, and dysfunction. An RVOT aneurysm is a ventricular wall swelling or its restored outflow, which is considered as an independent predictor of RV dilatation and systolic dysfunction in patients that have been subjected to an rToF. It is also a suitable substrate for the generation of ventricular arrhythmias. MDCT images clearly delineate the RVOT aneurysms as well as any associated dilatation of the main PA and its central branches. MDCT is excellent in depicting the morphology of the RVOT and cardiac abnormalities related to PR, in addition to accurate measurements of the enlarged RV volumes that serve as one of the major criteria for pulmonary valve replacement (2).
The present study had several limitations. First, this study was retrospective, and thereby limited by the small sample size. Additionally, being an observational study, it lacked gold standard for comparison.
CONCLUSION
Our study demonstrated that the most frequent complications observed in pediatric patients with repaired ToF were PA stenosis and PA branch stenosis. Patients with PA and PA branch stenosis exhibit no significant differences in terms of their age, anthropometric parameters (height, weight, and BSA), and gender distribution in the presence or absence of different stenosis types (PA, RPA, LPA). However, the presence of an RPA stenosis provides a greater chance of developing an LPA stenosis.
MDCT is an extremely useful imaging method for evaluating normal and abnormal findings after the surgical rToF due to its wide availability and high spatial and temporal resolution. MDCT is being used with increasing frequency to evaluate patients with ToF, as it provides reliable and accurate assessment of complex anatomy and associated anomalies in unrepaired ToF patients and guides the surgical approach and the necessary type of surgery needed. In addition, MDCT has given us the opportunity to fully understand and assess the late surgical sequelae, surgical complications, and residual lesions. Therefore, it is now essential that corrected ToF patients should have regular follow-ups in order to assess the existence of any residual lesions and surgical complications and to manage them appropriately and in a timely manner.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. R.I. Rakhimzhanova, T.B. Dautov ― conception and design of the work; A.M. Kabdullina, B.B. Kaliyev, L.A. Bastarbekova, Zh.A. Moldakhanova ― acquisition, analysis of data; A.M. Kabdullina, A.B. Saduakassova ― interpretation of data; V.E. Sinitsyn ― approved the final version of the work.
Acknowledgments. We thank the Chief of the National Research Cardiac Surgery Center MD Ph.D. professor Yuriy V. Pya for the opportunity to carry out this work.
About the authors
Azhar M. Kabdullina
Astana Medical University
Author for correspondence.
Email: azharazh@mail.ru
ORCID iD: 0000-0003-0521-5484
SPIN-code: 4169-1761
Kazakhstan, Astana
Valentin E. Sinitsyn
Lomonosov Moscow State University
Email: vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-code: 8449-6590
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowRaushan I. Rakhimzhanova
Astana Medical University
Email: rakhimzhanova01@rambler.ru
ORCID iD: 0000-0002-3490-6324
MD, Dr. Sci. (Med.), Professor
Kazakhstan, AstanaTairkhan B. Dautov
Department of the Radiology of National Research Cardiac Surgery Center
Email: tairkhan.dautov@mail.ru
ORCID iD: 0000-0002-5267-0108
MD, Dr. Sci. (Med.), Professor
Kazakhstan, AstanaAigul B. Saduakassova
Medical Centre Hospital of President’s Affairs Administration of the Republic of Kazakhstan
Email: sadik.a@mail.ru
ORCID iD: 0000-0001-7089-5696
MD, Dr. Sci. (Med.)
Kazakhstan, AstanaBaurzhan B. Kaliyev
Astana Medical University
Email: Baur233113@mail.ru
ORCID iD: 0000-0003-4825-749X
Kazakhstan, Astana
Lyazzat A. Bastarbekova
Astana Medical University
Email: lbastarbekova@mail.ru
ORCID iD: 0000-0001-8246-4754
SPIN-code: 8634-6601
Kazakhstan, Astana
Zhanar A. Moldakhanova
Astana Medical University
Email: moldahanova1991@mail.ru
ORCID iD: 0000-0002-5980-9563
Kazakhstan, Astana
References
- Apostolopoulou SC, Manginas A, Kelekis NL, Noutsias M. Cardiovascular imaging approach in pre and postoperative tetralogy of Fallot. BMC Cardiovasc Dis. 2019;19(1):7. doi: 10.1186/s12872-018-0996-9
- Shaaban M, Tantawy S, Elkafrawy F, et al. Multi-detector computed tomography in the assessment of tetralogy of Fallot patients: Is it a must? Egyptian Heart J. 2020;72(1):17. doi: 10.1186/s43044-020-00047-3
- Goo HW. Changes in right ventricular volume, volume load, and function measured with cardiac computed tomography over the entire time course of tetralogy of Fallot. Korean J Radiol. 2019;20(6):956–966. doi: 10.3348/kjr.2018.0891
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology / American Heart Association Task Force on Clinical Practice Guidelines. J Am College Cardiol. 2019;73(12):1494–1563. doi: 10.1016/j.jacc.2018.08.1028
- Chelliah A, Shah AM, Farooqi KM, Einstein AJ. Cardiovascular CT in cyanotic congenital heart disease. Curr Cardiovasc Imaging Rep. 2019;12(7):30. doi: 10.1007/s12410-019-9507-3
- Goo HW. Comparison of chest pain protocols for electrocardiography-gated dual-source cardiothoracic CT in children and adults: The effect of tube current saturation on radiation dose reduction. Korean J Radiol. 2018;19(1):23–31. doi: 10.3348/kjr.2018.19.1.23
- Siripornpitak S, Goo HW. CT and MRI for repaired complex adult congenital heart diseases. Korean J Radiol. 2021;22(3):308–323. doi: 10.3348/kjr.2020.0895
- Singh R, Jain N, Kumar S, Garg N. Multi-detector computed tomography angiographic evaluation of right ventricular outflow tract obstruction and other associated cardiovascular anomalies in tetralogy of Fallot patients. Polish J Radiol. 2019;84:511–516. doi: 10.5114/pjr.2019.91203
- Kossaify A. Echocardiographic assessment of the right ventricle, from the conventional approach to speckle tracking and three-dimensional imaging, and insights into the “Right Way” to explore the forgotten chamber. Clin Med Insights Cardiol. 2015;9:65–75. doi: 10.4137/CMC.S27462
- Pushparajah K, Duong P, Mathur S, Babu-Narayan SV. Cardiovascular MRI and CT in congenital heart disease. Echo Res Pract. 2019;6(4):R121–138. doi: 10.1530/ERP-19-0048
Supplementary files