Computed tomography in the diagnosis of fever of unknown origin: A case report
- Authors: Shumskaya Y.F.1, Kostikova N.V.2, Akhmedzyanova D.A.1, Suleymanova M.M.2, Fominykh E.V.2, Mnatsakanyan M.G.2, Reshetnikov R.V.1
-
Affiliations:
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- The First Sechenov Moscow State Medical University (Sechenov University)
- Issue: Vol 4, No 3 (2023)
- Pages: 393-402
- Section: Case reports
- Submitted: 02.06.2023
- Accepted: 04.07.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/472068
- DOI: https://doi.org/10.17816/DD472068
- ID: 472068
Cite item
Abstract
Fever of unknown origin can be a symptom of at least 200 diseases. Positron emission tomography-computed tomography, although highly informative, may not be readily available as an imaging tool. We present a clinical case of giant cell arteritis where computed tomography played a key role in arriving at a diagnosis.
A 61-year-old woman presented to the hospital with a nocturnal fever up to 39.5°С, accompanied by chest and scapular pain, and substantial weight loss (10 kg over 3 months). Lymphoproliferative and infectious diseases were excluded. Baseline colonoscopy had revealed erosions in the colonic mucosa, leading to a preliminary diagnosis of ulcerative colitis, and subsequently, the patient was admitted to the gastroenterology department. Follow-up colonoscopy had excluded this diagnosis. Additional imaging via chest and abdominal computed tomography scan revealed wall thickening of aorta and its branches with subtle contrast enhancement.
Conditions, such as tuberculous aortoarteritis and syphilitic aortitis, were excluded. The patient was diagnosed with giant cell arteritis involving brachiocephalic trunk, subclavian arteries, and celiac trunk. Prednisolone was administered with subsequent reduction in symptoms.
Although computed tomography may not be regarded as the gold standard for the differential diagnosis of fever of unknown origin, this case underscores its valuable contribution in establishing a definitive diagnosis.
Full Text
BACKGROUND
Over 200 disorders can produce fever of unknown origin (FUO) [1]. Infections, noninfectious inflammatory conditions (e.g., systemic lupus erythematosus and systemic vasculitis), and malignant disorders are the most common [2, 3]. Furthermore, up to 50% of FUO cases go untreated [4, 5]. Most clinicians find the diagnostic search challenging because the disorders that might induce FUO are so diverse. A time-consuming diagnostic search results in longer inpatient stays, which increases the risk of hospital-acquired infections and medical examination costs [6].
Diagnostic search and differential diagnosis procedures include positron emission tomography–computed tomography (PET/CT), computed tomography (CT), and ultrasound scan (US). The procedure is chosen depending on the symptoms and likely involvement of the systems and organs [7]. According to a retrospective study [4], PET/CT provided the best informative value in establishing the final diagnosis in 54% of patients with FUO. In contrast, detecting rheumatic diseases and infections with fever as the only symptom does not necessitate this costly and limited-available technology. Alternative imaging methods are more appropriate in this case.
This study presents a case of giant cell arteritis with an unusual course that did not affect the temporal artery. With a diagnosis of ulcerative colitis and a long-term fever, the patient was referred for additional diagnostics, and the decisive evidence for the final diagnosis was established with CT with intravenous contrast. This case report was prepared in line with CARE guidelines [8].
CASE REPORT
Patient
In November 2020, the 61-year-old female patient was admitted to the Gastroenterology Department of the Clinical Center at the Sechenov First Moscow State Medical University (Sechenov University UCH1) with complaints of general weakness, febrile body temperature up to 39.5°C in the evenings, pain in the pericardiac and interscapular areas, and a 10-kg weight loss over the previous 3 months.
Disease
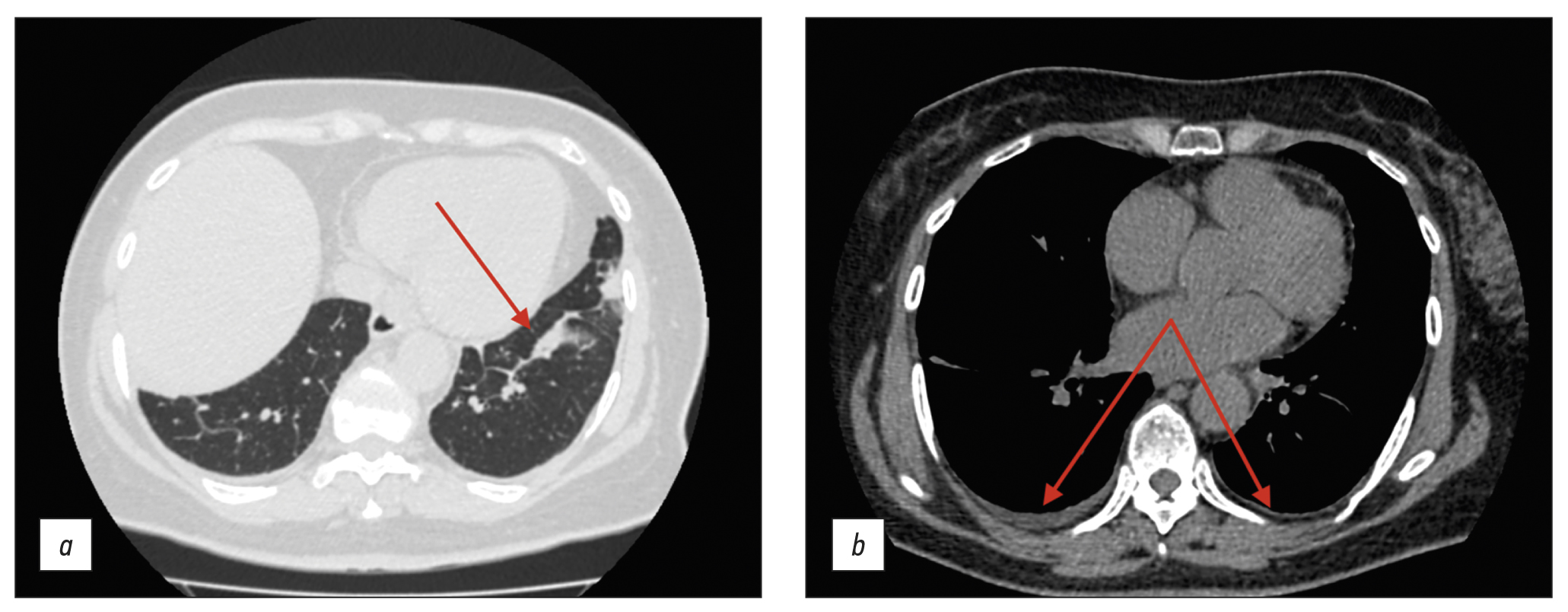
The patient was generally well in August 2020, but she began experiencing daily elevations in body temperature to 38.5°C–39°C, followed by muscular and joint pain. Antipyretics had no effect; therefore, the patient was admitted to the Infection Department inpatient unit and received antibiotics and oral and intravenous detoxification medications. The medication improved the overall condition, although the subfebrile body temperature continued in the evenings. The thoracic CT scan revealed modest effusion in the pleural cavities, bilateral bands of peribronchovascular thickening, small subsegmental compression atelectasis in the basal regions of both lungs, and elevated hemidiaphragm (Figure 1).
Fig. 1. Thoracic computed tomography scan (August 2020); axial plane: the red arrows show (a) subsegmental compression atelectasis and (b) mild pleural effusion.
Upper gastrointestinal tract esophagogastroduodenoscopy revealed no abnormalities. A colonoscopy revealed erosive lesions of the descending colon, sigmoid, and rectum mucosa. The histology of the colon biopsy samples revealed catarrhal colitis. Abdominal CT with intravenous contrast (Figure 2) showed several abdominal lymph nodes (para-aortic and superior mesenteric lymph nodes, up to 10-mm short-axis diameter) during the patient’s stay in the Infection Department inpatient unit.
Fig. 2. Abdominal computed tomography scan with intravenous contrast (September 2020); axial plane: the red arrows show intraperitoneal lymph nodes.
The findings were classified as lymphadenopathy, and the patient was referred to a hematologist in September 2020. A bone marrow trephine sample was conducted, followed by histological examination; no indication of the hematopoietic system was identified. Compared with the earlier scan in August 2020 (see Figure 1), follow-up thoracic CT (Figure 3) revealed effusion resorption from the pleural cavities and partial regress of hypoventilatory changes in the basal parts of the lungs. Otherwise, no significant changes were found.
Fig. 3. Thoracic computed tomography scans (September 2020); axial plane: the red arrows show (a) the area with partial regression of the hypoventilation changes and (b) lack of pleural effusion.
The patient was hospitalized in the inpatient unit several times in November 2020 for body temperature rises to 39°C in the evenings and copious nocturnal sweating. Ulcerative colitis was suspected as a cause of fever in the context of the colonoscopy results from August 2020. Due to the positive PCR test for COVID-19, the patient was discharged from the inpatient unit and advised to continue the examinations once she was convalescent. The patient was also given a mesalazine medication at 4 g/day for ulcerative colitis, which worked well. However, after experiencing persistent fevers, the patient terminated the product without visiting a doctor.
The patient was sent to a coloprotologist in November 2020 after two consecutive negative COVID-19 swabs; the outpatient examination revealed fecal calprotectin increased to 213 µm/g (N: max. 50 µm/g). The patient was hospitalized in the Gastroenterology Department of Sechenov University UCH1 for examination and a decision on further treatment tactics.
Physical examination, laboratory tests, and investigations
The primary physical examination in the department showed an increased body temperature of 37.5°C, pallor of the skin and visible mucosae, rales in the posterior basal part of the left lung, tachycardia at 98 bpm, and moderate abdominal tenderness in the periumbilical area. The peripheral pulses remained intact and adequate. The rest of the show was unimpressive.
The laboratory studies revealed a significant increase in nonspecific inflammatory markers (Table 1).
Table 1. Pretreatment inflammatory markers measured during hospitalization
Parameter | N | November 24, 2020 | December 01, 2020 |
Erythrocyte sedimentation rate (mm/h) | 1–20 | 71 | 55 |
Fibrinogen (g/L) | 1.8–4 | 10.16 | 10.97 |
C-reactive protein (mg/L) | 0–5 | 119 | 130 |
The abdominal US scan revealed no lymphadenopathy or inflammation in the liver, pancreas, and biliary ducts. The patient had a follow-up fibro colonoscopy, which showed that the colon and distal jejunum mucosae were in the same condition. The histological examination of the biopsy samples of the colon mucosae failed to find any evidence of structural and inflammatory changes.
Because there was no evidence of ulcerative colitis, the patient with FUO and high levels of nonspecific inflammatory markers was tested for the extractable nuclear antigens panel, which revealed no Jo-1, RNP/Sm, Scl-70, Sm, SS-A (Ro), SS-B (La), pANCA, or сANCA.
The follow-up thoracic and abdominal CT with i.v. contrast showed pleural thickening in the apical area of the lungs; aortic wall thickening up to 5 mm and lamellar, poorly defined contours of the aortic wall were remarkable, and they actively accumulated the contrast agent. Similar changes were visualized in the brachiocephalic trunk, subclavian arteries, and celiac artery walls. In addition, fibromuscular dysplasia of the renal arteries was discovered. The CT findings were consistent with active arteritis.
Fig. 4. Thoracic and abdominal computed tomography scan with i.v. contrast (November 2020): the red arrows show changes in the walls of the brachiocephalic trunk and subclavian arteries (а, axial plane); aortic wall thickening (b, axial plane); lamellar image if the aortic walls with contrast accumulation (с, sagittal plane); and occlusion of the celiac artery mouth (d, sagittal plane).
Diagnosis
Based on the findings, the differential diagnosis was carried out for giant cell arteritis or specific arteritis. Because of the patient’s age and atypical aortic lesion, Takayasu’s arteritis was overlooked. The syphilis serology test came out negative. The patient also consulted a tuberculosis specialist and had a T-SPOT.TB test, the result of which was consistent with functional incompetence of lymphocytes. A rheumatologist reviewed the examination results and diagnosed the patient with giant cell arteritis involving the aorta, brachiocephalic trunk, subclavian arteries, and celiac artery. Anti-inflammatory therapy was started with 60-mg/day prednisolone, which was co-administered with the antituberculosis medication of 0.3-g/day isoniazid + 0.03-g/day pyridoxine hydrochloride.
Changes over time and outcomes
The patient was advised to have 18F-FDG PET/CT to evaluate the length of arterial lesions, but she refused due to the significant clinical improvement with glucocorticoids.
A good response was noted while on therapy—the fever subsided; chest pain and overnight sweats did not return; and a trend toward normalized laboratory results was demonstrated (ESR ↓ to 42 mm/h; CRP ↓ to 16 mg/L; and fibrinogen ↓ to 6.76 g/L). Subjectively, the patient experiences improved general health and normalized appetite. At discharge, complaints of weakness and palpitations related to physical exertion or emotional distress persisted.
The patient had a follow-up appointment after 3 months. The general condition was satisfactory, with no complaints, and the therapy with 4-mg/day methylprednisolone was ongoing.
DISCUSSION
The case report highlights how challenging it is to establish the diagnosis in patients with large vessel involvement who mainly present with FUO. Our patient arrived with nonspecific symptoms, such as increased body temperature, weight loss, asthenia, autonomic nervous system problems, and pain syndrome, but she lacked characteristic temporal arteritis signs. Furthermore, the patient exhibited no signs and symptoms of vascular insufficiency, which could have suggested large vessel vasculitis, contributing to the delayed diagnosis. Moreover, abnormalities associated with ulcerative colitis were discovered during a colonoscopy and biopsy sample analysis, which was regarded as a likely cause of FUO and for which the patient was referred to a coloproctologist and was hospitalized in the Gastroenterology Department. However, the absence of gastrointestinal symptoms initially hampered the diagnosis of ulcerative colitis. Given the above, the patient had a follow-up colonoscopy, tissue samples were collected, and their analysis revealed no indication of colon inflammation.
The literature describes less than 10 cases of ulcerative colitis onset accompanied by isolated FUO without any relevant colon symptoms. A 71-year-old patient with ulcerative colitis was treated with FUO alone without any gastrointestinal problems. The final diagnosis was based on the PET/CT scan that revealed increased 18F-FDG accumulation in the colon walls, indicating active inflammation [9]. Soliman et al. [10] presented another example of Crohn’s disease, an inflammatory bowel disease (IBD), in a 29-year-old patient with long-term persistent fever and mild gastrointestinal symptoms. The thickening cecum wall was visible on the abdomen CT scan; the diagnosis was later confirmed by colonoscopy and tissue biopsy.
In our case report, we interpreted historical data on colon mucosa lesions as the outcome of reactive colitis as part of a systemic inflammatory response that did not originate in the colon. It was indicated by the resolution of inflammation with short-term therapy with 5-aminosalicylic acid products. Interestingly, some patients are diagnosed with an IBD after the diagnosis of giant cell arteritis has been established. A prospective study by Yavne et al. [11] showed that patients diagnosed with giant cell arteritis often have a secondary diagnosis of an IBD, such as Crohn’s disease or ulcerative colitis (hazard ratio: 2.63; p < 0.001). As a result, doctors should be on the lookout for the beginning of an IBD in patients with giant cell arteritis.
There were no studies that compared the value of CT with PET/CT in diagnosing FUO. CT provides several advantages, including shorter scan duration, high availability, a lower cost, and a lower radiation dose. In our case report, it was CT with i.v. contrast that provided decisive evidence for the final diagnosis. A similar case was described by Schäfer et al. [12]: a 79-year-old patient presenting with FUO, underweight, and elevated inflammatory markers underwent thoracic and abdominal CT with i.v. contrast, which showed thickened aortic walls and major aortic branch walls; the temporal artery was intact. The temporal artery remained unchanged despite thicker aortic walls and major aortic branch walls. Giant cell arteritis was diagnosed in the patient. Later, PET/CT was used to rule out cancer, and glucocorticoid medication was started, which had a beneficial effect. Al Nuaimi et al. [13] also described a 63-year-old patient with recurrent fever, rapid weight loss, and high inflammatory markers who unexpectedly developed recurrent vision loss episodes. He was referred to an inpatient care facility for a brain MRI, which revealed an MRI presentation compatible with an ischemic stroke of the right occipital area. Later, the patient had a thoracic and abdominal CT scan with i.v. contrast was visualized to identify the cause of FUO and diffuse thickening of the aortic walls and primary aortic branch walls with contrast accumulation. An ultrasound examination was performed, followed by a temporal artery tissue examination, because giant cell arteritis was suspected. As a result, giant cell arteritis involving the temporal artery was confirmed. The lesion length was measured by 18F-FDG PET/CT, and glucocorticoid medication was started, which had a positive effect [13]. However, CT may not always entirely or partially substitute PET/CT to diagnose giant cell arteritis. For example, Grazioli-Gauthier et al. [14] described giant cell arteritis with an atypical course, with FUO as the most prominent symptom. In a 73-year-old patient with fever and increased acute inflammatory markers, thoracic and abdominal CT was inconclusive; thus, PET/CT was performed. PET/CT revealed minimally active inflammation in the ascending aorta. Metabolic activity was visualized in the ascending colon (later colonoscopy ruled out bowel diseases). The temporal artery tissue investigation was used for the final diagnosis [14].
In contrast to the examples in the literature, where the diagnosis was based on PET/CT and/or temporal artery tissue examination, we relied on CT with i.v. contrast to diagnose giant cell arteritis. It was feasible due to the increased activity and spread of inflammation and the disease’s typical trajectory without temporal artery involvement.
CONCLUSION
Several key conclusions can be drawn from the case report. Giant cell arteritis may cause a fever of unknown origin (FUO) and have an unusual course without the temporal artery involvement, hindering the correct diagnosis. CT with intravenous contrast may aid in diagnosing arteritis, including giant cell arteritis with temporal artery involvement. In some cases, CT with intravenous contrast may be an alternative to PET/CT in the diagnostic search for the cause of a FUO.
ADDITIONAL INFORMATION
Funding source. This article was prepared by a group of authors as a part of the research and development effort titled “Opportunistic screening of high-profile and other common diseases”, No. 123031400009-1 (USIS No. 123031400009-1) in accordance with the Order No. 1196 dated December 21, 2022 “On approval of state assignments funded by means of allocations from the budget of the city of Moscow to the state budgetary (autonomous) institutions subordinate to the Moscow Health Care Department, for 2023 and the planned period of 2024 and 2025” issued by the Moscow Health Care Department.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Y.F. Shumskaya — concept, collection and processing of material, analysis of data, writing of the manuscript; N.V. Kostikova — collection and processing of material, writing of the manuscript; D.A. Akhmedzyanova — concept, manuscript editing; M.M. Suleymanova — manuscript editing, preparation of illustrative material; E.V. Fominykh — manuscript editing, preparation of illustrative material; M.G. Mnatsakanyan — final editing of the manuscript. Suleymanova — manuscript editing, preparation of illustrativaration of illustrative material; M.G. Mnatsakanyan — final editing, manuscript approval, R.V. Reshetnikov — manuscript writing, final editing.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics Journal.
Acknowledgments. The authors would like to thank Ivan Andreevich Blokhin for his help with drafting this article.
About the authors
Yuliya F. Shumskaya
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Author for correspondence.
Email: ShumskayaYF@zdrav.mos.ru
ORCID iD: 0000-0002-8521-4045
SPIN-code: 3164-5518
Russian Federation, Moscow
Nina V. Kostikova
The First Sechenov Moscow State Medical University (Sechenov University)
Email: n.kostikowa@yandex.ru
ORCID iD: 0000-0003-3509-7271
SPIN-code: 7962-4554
Russian Federation, Moscow
Dina A. Akhmedzyanova
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: dina_akhm@mail.ru
ORCID iD: 0000-0001-7705-9754
SPIN-code: 6983-5991
Russian Federation, Moscow
Maria M. Suleymanova
The First Sechenov Moscow State Medical University (Sechenov University)
Email: ashe.danny.jush@gmail.com
ORCID iD: 0000-0002-5776-2693
SPIN-code: 7193-6122
Russian Federation, Moscow
Ekaterina V. Fominykh
The First Sechenov Moscow State Medical University (Sechenov University)
Email: evfominykh@mail.ru
ORCID iD: 0000-0003-3733-4381
Cand. Sci (Med.), Head of the Radiology Department
Russian Federation, MoscowMarina G. Mnatsakanyan
The First Sechenov Moscow State Medical University (Sechenov University)
Email: mnatsakanyan08@mail.ru
ORCID iD: 0000-0001-9337-7453
SPIN-code: 2015-1822
MD, Dr. Sci. (Med), Professor
Russian Federation, MoscowRoman V. Reshetnikov
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: reshetnikov@fbb.msu.ru
ORCID iD: 0000-0002-9661-0254
SPIN-code: 8592-0558
Cand. Sci. (Phys.-Math.)
Russian Federation, MoscowReferences
- Unger M, Karanikas G, Kerschbaumer A, et al. Fever of unknown origin (Fuo) revised. Wien Klin Wochenschr. 2016;128(21-22):796–801. doi: 10.1007/s00508-016-1083-9
- David A, Quinlan JD. Fever of unknown origin in adults. Am Fam Physician. 2022;105(2):137–143.
- Fusco FM, Pisapia R, Nardiello S, et al. Fever of unknown origin (FUO): Which are the factors influencing the final diagnosis? A 2005–2015 systematic review. BMC Infect Dis. 2019;19(1):653. doi: 10.1186/s12879-019-4285-8
- Weitzer F, Hooshmand T, Pernthaler B, et al. Diagnostic value of F-18 FDG PET/CT in fever or inflammation of unknown origin in a large single-center retrospective study. Sci Rep. 2022;12(1):1883. doi: 10.1038/s41598-022-05911-7
- Wright WF, Auwaerter PG. Fever and fever of unknown origin: Review, recent advances, and lingering dogma. Open Forum Infect Dis. 2020;7(5):132. doi: 10.1093/ofid/ofaa132
- Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med. 2013;368(3):197–199. doi: 10.1056/NEJMp1212725
- Cunha BA, Lortholary O, Cunha CB. Fever of unknown origin: A clinical approach. Am J Med. 2015;128(10):1138.e1–1138.e15. doi: 10.1016/j.amjmed.2015.06.001
- Barber MS, Aronson JK, von Schoen-Angerer T, et al. CARE guidelines for case reports: explanation and elaboration document. Translation into Russian. Digital Diagnostics. 2022;3(1):16–42. (In Russ). doi: 10.17816/DD105291
- Shpilberg R, Hadjiyiannis D, Khan SA. Ulcerative colitis presenting as pyrexia of unknown origin (PUO) without bowel symptoms. Clin Med (Lond). 2012;12(4):389–390. doi: 10.7861/clinmedicine.12-4-389
- Soliman M, Shirazi-Nejad A, Bullas D, et al. An unusual case of pyrexia of unknown origin. Cureus. 2021;13(7):e16684. doi: 10.7759/cureus.16684
- Yavne Y, Tiosano S, Ben-Ami D, et al. Giant cell arteritis and inflammatory bowel disease: Is there a connection? Results from a population-based study. Autoimmun Rev. 2018;17(11):1134–1137. doi: 10.1016/j.autrev.2018.06.003
- Schäfer VS, Warrington KJ, Williamson EE, Kermani TA. Delayed diagnosis of biopsy-negative giant cell arteritis presenting as fever of unknown origin. J Gen Intern Med. 2009;24(4):532–536. doi: 10.1007/s11606-009-0925-9
- AlNuaimi D, Ansari H, Menon R, et al. Large vessel vasculitis and the rising role of FDG PET-CT: A case report and review of literature. Radiol Case Rep. 2020;15(11):2246–2249. doi: 10.1016/j.radcr.2020.08.066
- Grazioli-Gauthier L, Marcoli N, Vanini G, et al. Giant cell arteritis among fevers of unknown origin (FUO): An atypical presentation. Eur J Case Rep Intern Med. 2021;8(3):002254. doi: 10.12890/2021_002254
Supplementary files