Cardiac magnetic resonance imaging in patients with history of COVID-19
- Authors: Maksimova A.S.1, Ryumshina N.I.1, Shelkovnikova T.A.1, Mochula O.V.1, Anfinogenova N.D.1, Ussov V.Y.1
-
Affiliations:
- Tomsk National Research Medical Center, Cardiology Research Institute
- Issue: Vol 4, No 3 (2023)
- Pages: 280-291
- Section: Original Study Articles
- Submitted: 16.06.2023
- Accepted: 10.07.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/494103
- DOI: https://doi.org/10.17816/DD494103
- ID: 494103
Cite item
Abstract
BACKGROUND: Myocarditis is among the most common complications arising from coronavirus infection (COVID-19).
AIM: This study aims to find the differences in the patterns of myocardial injury between patients who had COVID-19 and those from the pre-pandemic period, as determined by contrast-enhanced cardiac magnetic resonance imaging.
MATERIALS AND METHODS: The study encompassed a retrospective analysis of 47 patients who underwent contrast-enhanced cardiac magnetic resonance imaging to rule out acute myocarditis. Group 1 comprised 34 patients with a confirmed history of COVID-19 through PCR testing (nasal and/or throat swabs), while Group 2 comprised 13 individuals who underwent contrast-enhanced cardiac magnetic resonance imaging in 2017 prior to the onset of the COVID-19 pandemic. All patients enrolled in the study had clinical manifestation of cardiac injury without signs of coronary artery disease as an underlying cause of condition.
RESULTS: The mean time from the onset of heart symptoms to the administration of contrast-enhanced cardiac magnetic resonance imaging was 166 days. In group 1, a decrease in exercise tolerance was observed in 77% of patients, and 14 (42%), 30 (88%), and 28 (85%) of patients complained of chest pain, shortness of breath, and heart palpitations, respectively. In group 2, four patients (30%) had dyspnea, nine patients (69%) complained of chest pain, and six patients (46%) had heart palpitations and/or feeling of arrhythmia. Myocardial injury in group 1 was more generalized. The third of them had displayed preserved increased pulmonary vascularity and pleural effusion. Within group 1, men had significantly lower left ventricular ejection fraction, lower values of global longitudinal deformation, and higher values of left atrial function compared with the corresponding parameters in women. Differences in women were found only in the number of the affected segments in the left ventricular myocardium.
CONCLUSION: SARS-CoV-2 virus caused extended myocardial injury, affecting a significant number of myocardial segments. Men had more frequent postinflammatory complications, characterized by abnormal function of the left ventricle and left atrium. Obtained results require continuous efforts for further assessment of long-term consequences of previous COVID-19 to the cardiovascular system. In this regard, contrast-enhanced cardiac magnetic resonance imaging may represent a sensitive imaging tool for the assessment of cardiac injury severity.
Full Text
Abbreviations
BSA body surface area
EDV end-diastolic volume
EF ejection fraction
ESV end-systolic volume
ESVi end-systolic volume index
LA left atrium
LAV left atrial volume
LAVi left atrium volume index
LGE late gadolinium enhancement
LV left ventricle
MRI magnetic resonance imaging
BACKGROUND
Since March 2020, COVID-19, caused by SARS-CoV-2 infection, has been declared a global pandemic. COVID-19 primarily affects the respiratory system [1], and treatment is aimed mainly at respiratory complications. On the other hand, the new coronavirus infection has a major influence on the cardiovascular system, particularly in patients with pre-existing cardiovascular diseases [2]. COVID-19 can cause myocardial injury at any stage of the infection, including the viral, pulmonary, inflammatory, and recovery phases and later stages after symptoms appear [3]. COVID-19 patients have been linked to arrhythmia, cardiac failure, and myocarditis [4]. The mechanisms of cardiac damage caused by SARS-CoV-2 infection are not entirely known.
The most common complication of coronavirus infection is myocarditis. Myocarditis manifests clinically as modest symptoms, such as fatigue and dyspnea, to rapid disease progression with heart failure and cardiogenic shock [5]. Imaging is critical for assessing many elements of myocardial damage, allowing for a precise diagnosis and early treatment. Contrast-enhanced cardiac magnetic resonance imaging (MRI) is recommended by the Russian and European Societies of Cardiology as an insightful, non-invasive method of imaging diagnosis in myocarditis, ensuring detailed visualization of anatomical structures and assessment of functional heart disorders [6, 7].
According to the World Health Organization, COVID-19 is no longer classified as a global pandemic. However, the pandemic’s long-term negative, harmful ramifications, including major cardiovascular issues, are only beginning to emerge. Thus, cardiac viability testing in post-COVID-19 patients is still important and warrants further research [8]. Furthermore, differences in study designs and inclusion/exclusion criteria, as well as imaging methodologies and data analysis, interpretation, and reporting in terms of changes in cardiac MRI findings, all contribute to high degree of variability in published study results.
The study compared different patterns of myocardial injury in post-COVID-19 patients to prepandemic patients using contrast-enhanced cardiac MRI data.
MATERIALS AND METHODS
Study design
This retrospective study was conducted in accordance with Good Clinical Practice and the principles described in the Helsinki Declaration.
Study conditions
The study was performed in the Department of X-ray and Imaging Diagnosis, Cardiology Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences. All patients provided informed consent to paramagnetic contrast-enhanced cardiac MRI.
Eligibility criteria
Inclusion criteria for Group 1: a history of SARS-CoV-2 infection confirmed by polymerase chain reaction testing, no symptoms of acute respiratory infection at the time of cardiac MRI, a negative polymerase chain reaction test for COVID-19, objective evidence of symptomatic cardiac injury, without signs of ischemic heart disease (chest pain/discomfort, palpitations, and dyspnea), and a mean time from the onset of complaints to MRI 166 ± 17.
Inclusion criteria for Group 2: objective evidence of symptomatic cardiac damage without indicators of ischemic heart disease (chest pain/discomfort, palpitations, and dyspnea) and cardiac MRI performed before the COVID-19 pandemic (2017).
Exclusion criteria for both groups included a history of myocardial infarction and low-quality cardiac MRI scans, which made analysis challenging.
Cardiac MRI protocol
The Vantage Titan MRI Scanner (Toshiba, Japan) was used to perform paramagnetic contrast-enhanced cardiac MRI and ECG- and respiratory-gated 1.5T MRI. Short- and long-axis MRI images of the myocardium were acquired before and after contrast enhancement. As a paramagnetic contrast agent, 0.5 M Gadobutrol was administered intravenously at a 0.1-mL/kg body weight dose. The slice thickness was 10 mm, with no gaps, and the data were recorded to a 256 × 256 matrix. T1- and T2-weighted sequences and a fat suppression sequence were used to assess the myocardium; dynamic SSFP sequences were used to determine the left ventricular (LV) volume and function; and gradient inversion-recovery sequences (GR-IR) were used to identify abnormal contrast uptake areas. The time of inversion (TI) was selected individually (mean TI = 300 ± 10 ms). The approved 17-segment model of localization diagnosis for LV myocardium was used to analyze areas of defective myocardium.
Cardiac MRI scans were examined in the Medical Genomics Resource Sharing Center using the cvi42 program (Circle Cardiovascular Imaging, Calgary, Canada). The Lake–Louise criteria were used for the diagnosis of myocarditis. Major criteria included edema, hyperemia, and regional fibrosis, and minor criteria included pericardial effusion or hyperintensive signal from the pericardium and LV wall motion abnormality [9]. On T2-weighted images (T2WI), the edema ratio (ER) was calculated as the myocardial-to-skeletal muscle signal intensity ratio. ER >2.0 was considered a symptom of edema. On T1WI, the relative paramagnetic contrast uptake (hyperemia) was assessed in inferolateral LV segments, which are most commonly affected by inflammatory changes. A relative paramagnetic contrast uptake of more than >4.0 was considered a symptom of hyperemia.
The presence and nature (subendocardial, subepicardial, or intramural) of late gadolinium enhancement (LGE) and the number of involved segments were assessed. The outlines of the endocardium and epicardium were used to automatically identify the functional characteristics of the left and right ventricles. End-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction (EF), left ventricular global radial and longitudinal strain, and minimum and maximum left atrial volume were among the functional parameters measured. The following parameters were calculated based on these measurements:
- left atrium volume index (LAVi, mL/m2) = LA volume/BSA, where LA = left atrium; BSA = body surface area;
- end-diastolic volume index (EDVi): EDVi = EDV/BSA;
- end-systolic volume index (ESVi): ESVi = ESV/BSA.
LA functional parameters were calculated as follows:
- LA ejection fraction (LAEF) = ([LAVmax − LAVmin]/LAVmax) × 100%;
- LA strain = ([LAVmax − LAVmin)/LAVmin) × 100% [10].
Furthermore, pericardial effusion, pleural effusion, and enhanced pulmonary vascularity were also evaluated.
Statistical processing
Statistical analysis was performed using STATISTICA 10 software. Absolute (n) and relative (%) frequencies are used to represent categorical variables. The mean (m) and standard deviation (SD) or median (Me) and interquartile range [Q1; Q3] are used to represent continuous variables. The Shapiro–Wilk test was used to determine the normality of distribution. For categorical variables, the unpaired t test (for normal distribution) or Mann–Whitney U test (for nonnormal distribution) was used, and for continuous variables, the chi-squared test was used. P < 0.05 was considered significant.
RESULTS
Study subjects
A contrast-enhanced cardiac MRI was done on 47 patients to rule out acute myocarditis. The first group consisted of 34 individuals with a coronavirus infection history verified by polymerase chain reaction testing of a nasopharyngeal and/or oropharyngeal swab. Before the COVID-19 pandemic (2017), a cardiologist referred 13 patients for cardiac MRI. Table 1 shows the clinical characteristics of patients and cardiac MRI parameters in the study group. The study and control groups were balanced by sex, age, and body mass index. In Group 1, 77% of patients reported lower exercise tolerance, whereas 14 (42%), 30 (88%), and 28 (85%) felt chest discomfort, dyspnea, and palpitations, respectively. In Group 2, four (30%) patients complained of dyspnea, nine (69%) reported chest pain, and six (46%) reported palpitations.
Table 1. Clinical characteristics and cardiac MRI parameters in post-COVID-19 patients and control individuals
Parameter | Group 1 COVID-19(+) n = 34 | Group 2 COVID-19(−) n = 13 | p |
Age, year | 62.5 [55; 66] | 52 [45; 65] | 0.07 |
Male, n (%) | 12 (35.3) | 6 (46.1) | 0.49 |
Body mass index, kg/m2 | 30.69±5.22 | 27.75±4.05 | 0.08 |
Body surface area, m2 | 1.95±0.27 | 1.97±0.17 | 0.15 |
Heart rate, bpm | 72.73±8.75 | 78±21.6 | 0.23 |
Concomitant diseases, n (%): • Hypertension • Diabetes mellitus • Ischemic heart disease • Chronic obstructive pulmonary disease | 17 (50) 2 (5) 8 (23) 9 (26) | 7 (53) 0 4 (30) 1 (7) | 0.81 0.37 0.61 0.16 |
Cardiac symptoms, n (%): • Chest pain • Palpitations • Dyspnea • Decreased exercise tolerance | 14 (42) 30 (88) 28 (85) 26 (77) | 4 (30) 9 (69) 6 (46) 5 (39) | 0.51 0.12 0.01* 0.01* |
LVEF, % | 55.07±19.34 | 63.31±4.9 | 0.14 |
EDV LV, mL | 113 [94.7; 153.8] | 135.6 [116.72; 167.79] | 0.98 |
ESV LV, mL | 41 [28.9; 83] | 50.87 [46.85; 63.04] | 0.31 |
EDVi LV, mL/m2 | 61.5 [48.2; 72.6] | 69.5 [62.1; 79.9] | 0.13 |
ESVi LV, mL/m2 | 22.7 [16.4; 45.7] | 26.1 [23.1;30.6] | 0.39 |
ER | 1.5±0.36 | 1.58±0.39 | 0.54 |
Edema (visual), n (%) | 5 (14.7) | 0 (0) | 0.14 |
LGE, n (%) | 33 (97) | 12 (92.3) | 0.47 |
Number of LGE segments | 6.79±2.36 | 3.25±1.48 | 0.000* |
Pericardial effusion, n (%) | 22 (64.7) | 5 (38) | 0.10 |
Pleural effusion, n (%) | 10 (29.4) | 0 (0) | 0.03* |
Increased pulmonary vascularity, n (%) | 12 (35.3) | 0 (0) | 0.01* |
GRS LV, % | 17.52±9.61 | 16.86±5.54 | 0.82 |
GLS LV, % | -10.51±5.49 | -10.96±2.66 | 0.78 |
Contrast enhancement index on T1-WI of the basal inferolateral segment | 1.54±0.29 | 1.71±0.53 | 0.17 |
Contrast enhancement index on T1-WI of the medial inferolateral segment | 1.53±0.29 | 1.51±0.5 | 0.89 |
Contrast enhancement index on T1-WI of the apical lateral segment | 1.55±0.34 | 1.38±0.33 | 0.13 |
LAV max, mL | 72.39 [56.3; 110.15] | 57.80 [51.84; 96.6] | 0.32 |
LAV min, mL | 35.44 [18.47; 62.09] | 21.35 [19.0; 42.07] | 0.49 |
RAV max, mL | 35.205 [31.08;53.11] | 40.2 [31.8; 51.095] | 0.68 |
RAV min, mL | 66.47 [55.96; 96.75] | 84.945 [66.14; 93.305] | 0.30 |
LAVi, mL/m2 | 39.13 [32.09; 51.09] | 27.8 [27.2; 43.89] | 0.17 |
LAEF, % | 53.55 [37.26; 163.32] | 61.22 [55.35; 64.51] | 0.13 |
LA strain, % | 115.28 [59.39; 163.32] | 149.7 [121.74; 173.93] | 0.30 |
Note. The data are presented as the median (Me) and interquartile range [Q1; Q3].
Abbreviations: EDV, end-diastolic volume; ESV, end-systolic volume; EDVi, end-diastolic volume index; ER, edema ratio; ESVi, end-systolic volume index; GRS LV, global radial strain left ventricular; LA strain, left atrial strain; LAEF, left atrial ejection fraction; LAV, left atrial volume; LAVi, left atrium volume index; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; RAV, right atrium volume; T1-WI, T1-weighted images.
*p < 0.05.
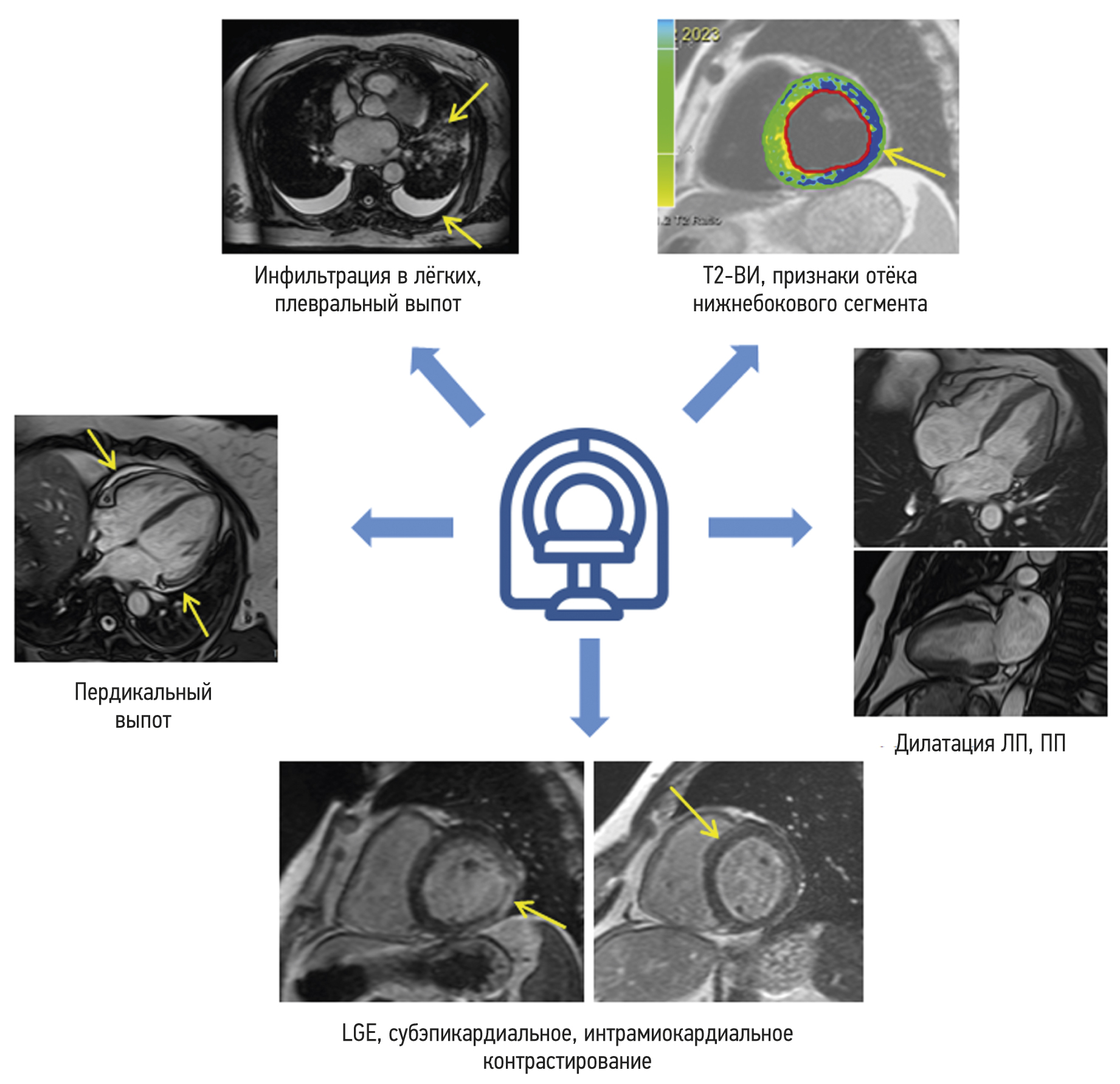
In the early phase of contrast enhancement (1–2 min), there was no difference between the groups in LV and LA functional metrics or contrast uptake. Group 1 patients demonstrated more severe myocardial injury, with more segments showing a nonischemic pattern of LGE. Furthermore, pulmonary changes (i.e., increased pulmonary vascularity and pleural effusion) remained in one-third of Group 1 patients during cardiac MRI (Figure 1).
Fig. 1. Characteristic symptoms detected on contrast-enhanced cardiac MRI in the group of post-COVID-19 patients. LA, left atrium; LGE, late gadolinium enhancement; RA, right atrium; T2WI, T2-weighted image.
Male patients in Group 1 exhibited considerably lower LVEF, lower global longitudinal strain, and higher LA functional characteristics, according to an intragroup comparison by sex. Only the number of affected LV myocardium segments differed significantly in female patients (Table 2). Male and female groups had no age differences (p = 0.78 and p = 0.18, respectively).
Table 2. Comparative analysis of the left ventricular and left atrial function based on MRI findings
Parameter | Men | Women | ||||
COVID-19(+) | COVID-19(−) | p | COVID-19(+) | COVID-19(−) | p | |
LVEF, % | 38 [26.2; 54.5] | 63 [62.5; 63.9] | 0.04* | 65 [59; 70] | 64.5 [60.5; 67.7] | 0.78 |
Number of LGE segments | 7.5 [6.5; 9.5] | 4 [3; 4] | 0.03* | 6.5 [5; 8] | 3 [2; 4] | 0.00* |
Longitudinal strain | -7.0 [-9.3; -3.1] | -10.2 [-11.1; -8.2] | 0.04* | -14.6 [-15.8; -10.2] | -10.9 [-15; -9.3] | 0.62 |
LA volume, min., mL | 83.8 [37.3; 127.8] | 25.6 [18; 35.3] | 0.02* | 25 [16.1; 35.9] | 21.4 [20; 48.9] | 0.59 |
LA ejection fraction, % | 30.1 [12.1; 47.8] | 63.5 [54.9; 68.5] | 0.01* | 59.9 [53.4; 72.2] | 59.9 [55.3; 64.5] | 0.94 |
LA strain, % | 43.1 [13.9; 91.9] | 173.8 [121.7; 217.9] | 0.01* | 149.9 [114.7; 259.7] | 143.7 [101; 157.9] | 0.43 |
Note. The data are presented as the median (Me) and interquartile range [Q1; Q3].
Abbreviations: LA, left atrium; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
*p < 0.05.
Key study findings
Our study found that post-COVID-19 patients had a higher number of affected LV myocardium segments based on delayed paramagnetic contrast uptake (a sign of fibrotic changes) than prepandemic patients with suspected myocarditis; however, the myocardial injury was nonspecific. Male patients showed lower LVEF, lower LV global longitudinal strain, and higher LV volume with reduced LV contractility. Because the SARS-CoV-2 virus primarily affects the respiratory system, the heterogeneous lung tissue thickening, and pleural effusion observed in post-COVID-19 patients were believed to represent evidence of long-term recovery from a respiratory infection. The control group did not show any of these changes.
DISCUSSION
Previous research [11] shows that the most prevalent COVID-19 symptoms are dyspnea and chest discomfort. Unfortunately, these symptoms remain even in patients with a negative SARS-CoV-2 test, resulting in a chronic COVID-19 syndrome. The long-term risks and clinical significance of these symptoms are not fully understood. The clinical indications found in this group of patients were considered to be the outcome of continuous myocardial injury caused by inflammation. However, most of our patients did not have myocardial edema, and the myocardium-to-muscle tissue signal intensity ratio on T2WI was within normal limits. Furthermore, there were no statistically significant differences in the increase in myocardium signal intensity within the first few minutes after the contrast injection. Thus, our sample lacked two of the three major MRI criteria for myocarditis (edema and hyperemia), preventing a definite diagnosis of myocarditis based on current guidelines [12].
In the study by Feofanova, evaluating the cardiovascular system in 36 patients with a history of acute COVID-19, there was a significant increase in LA volume with preserved LVEF. Only 8.4% of patients had a decline in LA systolic function, whereas 83.3% had a rise in LV volume [13]. Furthermore, the majority of study participants had LV myocardial hypertrophy (94.4%), pulmonary hypertension (72.2%), and atrial and ventricular ectopic activity manifested by supraventricular arrhythmia (94.4%), ventricular arrhythmia (63.9%), and paroxysmal supraventricular tachycardia (36.1%). The decreased heart function observed in our study was not statistically significant. The mean LVEF was 55%, with a median EDV of 61.5 mL/m2. LAVi in Group 1 exceeded 39.13 mL/m2, whereas LAVi in Group 2 was 27.8 mL/m2. The LV wall thickness in our sample was within normal limits, and pulmonary alterations remained in one-third of post-COVID-19 patients. There were no cases of rhythm disturbance (a common post-COVID-19 complication) in our sample.
According to some studies, the earlier indicator of LV myocardium remodeling is a decrease in LV longitudinal and/or global strain [14]. The LV longitudinal strain was reduced in both groups, although there were no significant differences between them. In our study, 21% of the patients (mainly men) had decreased LV contractility.
Our data indicating gender inequalities between groups are significant. As previously stated, Group 1 patients, both male and female, showed more severe LV myocardial injury in terms of the number of segments with fibrotic changes (p < 0.0000). A comparison of LAVi, LAEF, and LA strains revealed no significant differences. However, when adjusted for sex, the LA strain and LAEF were significantly lower in male post-COVID-19 patients.
LA volume reflects LV filling pressure and, consequently, the degree of LV diastolic dysfunction [2, 3]. According to some studies, an increase in LA volume is related to an increased risk of atrial fibrillation [15–17] and thromboembolism if it is accompanied by dysfunction in atrial fibrillation patients [18]. Changes in LA volume index and LAEF in patients with heart failure with intact ejection fraction and sinus rhythm are independently related to poor cardiovascular outcomes, comparable with those in persistent atrial fibrillation when LA measures have no predictive value [19].
The evidence on gender differences in LA remodeling, particularly in post-COVID-19 patients, is limited. Chistyakova et al. [20] found an increase in LAVi in all study groups compared with the control group in their cardiac function and endothelial dysfunction research in post-COVID-19 patients. According to some authors, LA remodeling is more prevalent in women. In the group of patients with recurrent atrial fibrillation with hypotension, for example, women exhibited a substantially lower LAEF than men (39% [28; 50] vs. 50% [42; 55], p = 0.02) [10]. In women with atrial fibrillation, LA diameter has been shown to be an independent predictor of cardiovascular death (p = 0.003) [21, 22]. According to EchoCG performed in post-COVID-19 patients 1 year after discharge, LAEF was significantly lower in the group with a decrease in LV global longitudinal strain (1.3 ± 0.3 vs. 1.4 ± 0.3 mL/m2; p = 0.052) [23]. Men have a higher incidence of atrial fibrillation regardless of COVID-19, whereas women have more evident atrial remodeling on high-density electroanatomic mapping and a higher incidence of arrhythmia recurrences after atrial fibrillation ablation. These modifications could explain why women have a higher incidence of recurrence and contribute to gender differences in the clinical course of atrial fibrillation [24]. Gender differences in atrial changes in post-COVID-19 patients require further research because they may be clinically significant for preventing atrial fibrillation and arrhythmia recurrences after atrial fibrillation ablation.
LA remodeling may be caused by a strong immune response, persistent inflammation, [25] endothelial damage, and microvascular thrombogenicity [26]. The possibility of virus retention in cardiomyocytes, resulting in fibrotic changes, cannot be completely ruled out.
The absence of a uniform approach to study design, which results in variability of study groups and makes study findings difficult to compare, can explain contradictory findings by different authors. However, it is clear that post-COVID-19 pneumonia patients with increased LAVi, LAEF, and LA strain with preserved LVEF require close monitoring to prevent or timely detection of complications, such as heart failure, LV dysfunction, and arrhythmia.
Limitations of the study
Each patient in our study had one cardiac MRI after the onset of symptoms. As a result, we cannot be certain that the observed findings did not exist prior to SARS-CoV-2 infection. Notably, the nonischemic LGEs found could be nonspecific and caused by undetected myocarditis before SARS-CoV-2 infection.
CONCLUSION
The SARS-CoV-2 virus unquestionably causes a more severe cardiac injury involving a greater number of myocardial segments. Residual effects of COVID-19 pneumonia, such as heterogeneous lung tissue thickening and pleural effusion, persist for longer. Male patients had a much higher prevalence of postinflammatory sequelae, manifesting as decreased LV and LA contractility.
Our findings highlight the need for further research into the long-term cardiovascular complications of COVID-19. In this case, contrast-enhanced cardiac MRI can be a sensitive imaging tool for evaluating the severity of cardiac injury.
ADDITIONAL INFORMATION
Funding source. This research was supported by the Russian Science Foundation (project #22-15-00313).
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. A.S. Maksimova, N.I. Ryumshina, T.A. Shelkovnikova — concept and design of the study, performance and analysis of cardiac MRI, drafting of the clinical conclusion, drafting of the text; O.V. Mochula, V.Y. Usov — performance and analysis of cardiac MRI, drafting of the clinical conclusion; N.D. Anfinogenova — concept and design of the study, drafting of the English version of the manuscript, general guidance.
About the authors
Aleksandra S. Maksimova
Tomsk National Research Medical Center, Cardiology Research Institute
Email: asmaximova@yandex.ru
ORCID iD: 0000-0002-4871-3283
SPIN-code: 2879-9550
MD, Cand. Sci. (Med.)
Russian Federation, TomskNadezhda I. Ryumshina
Tomsk National Research Medical Center, Cardiology Research Institute
Author for correspondence.
Email: n.rumshina@list.ru
ORCID iD: 0000-0002-6158-026X
SPIN-code: 6555-8937
MD, Cand. Sci. (Med.)
Russian Federation, TomskTatiana A. Shelkovnikova
Tomsk National Research Medical Center, Cardiology Research Institute
Email: fflly@mail.ru
ORCID iD: 0000-0001-8089-2851
SPIN-code: 1826-7850
MD, Cand. Sci. (Med.)
Russian Federation, TomskOlga V. Mochula
Tomsk National Research Medical Center, Cardiology Research Institute
Email: mochula.olga@gmail.com
ORCID iD: 0000-0002-7502-7502
SPIN-code: 3712-8492
MD, Cand. Sci. (Med.)
Russian Federation, TomskNina D. Anfinogenova
Tomsk National Research Medical Center, Cardiology Research Institute
Email: cardio.intl@gmail.com
ORCID iD: 0000-0003-1106-0730
SPIN-code: 6784-5440
MD, Dr. Sci. (Med.)
Russian Federation, TomskVladimir Yu. Ussov
Tomsk National Research Medical Center, Cardiology Research Institute
Email: ussov1962@yandex.ru
ORCID iD: 0000-0002-7352-6068
SPIN-code: 1299-2074
MD, Dr. Sci. (Med.), Professor
Russian Federation, TomskReferences
- Ussov WY, Nudnov NV, Ignatenko GA, et al. Primary and prospective imaging of the chest using magnetic resonance imaging in patients with viral lung damage in COVID-19. Medical Imaging. 2020;24(4):11–26. (In Russ). doi: 10.24835/1607-0763-2020-4-11-26
- Srinivasan A, Wong F, Couch LS, Wang BX. Cardiac complications of COVID-19 in low-risk patients. Viruses. 2022;14(6):1322. doi: 10.3390/v14061322
- Cosyns B, Lochy S, Luchian ML, et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21(7):709–714. doi: 10.1093/ehjci/jeaa136
- Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004
- Luetkens JA, Isaak A, Öztürk C, et al. Cardiac MRI in suspected acute COVID-19 myocarditis. Radiol Cardiothorac Imaging. 2021;3(2):e200628. doi: 10.1148/ryct.2021200628
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557
- Ferreira VM, Plein S, Wong TC, et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: Recommendations by the society for cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2023;25(1):21. doi: 10.1186/s12968-023-00933-0
- Yong SJ. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397
- Lewis AJ, Burrage MK, Ferreira VM. Cardiovascular magnetic resonance imaging for inflammatory heart diseases. Cardiovascular Diagnosis Therapy. 2020;10(3):598–609. doi: 10.21037/cdt.2019.12.09
- Kokhan EV, Ozova M., Romanova VA, et al. Left atrial phasic function in patients with hypertension and recurrent atrial fibrillation: Gender differences of the relationship with diastolic dysfunction and central aortic pressure. Rational Pharmacotherapy Cardiology. 2019;15(5):622–633. (In Russ). doi: 10.20996/1819-6446-2019-15-5-622-633
- Kravchenko D, Isaak A, Zimmer S, et al. Cardiac MRI in patients with prolonged cardiorespiratory symptoms after mild to moderate COVID-19. Radiology. 2021;301(3):E419–E425. doi: 10.1148/radiol.2021211162
- Arutyunov GP, Paleev FN, Moiseeva OM, et al. 2020 Clinical practice guidelines for myocarditis in adults. Russ J Cardiol. 2021;26(11):4790. (In Russ). doi: 10.15829/1560-4071-2021-4790
- Feofanova TB, Zaletova TS, Abakarov RM, Zainudinov ZM. Assessment of the state of the cardiovascular system in patients with COVID-19. Int J Med Psychol. 2021;4(7):84–87. (In Russ).
- Shirokov NE, Yaroslavskaya EI, Krinochkin DV, et al. Relationship between latent left ventricular contractile dysfunction and signs of immune inflammation in patients with COVID-19 pneumonia. Cardiovascular Therapy Prevention. 2023;22(3):3434. (In Russ). doi: 10.15829/1728-8800-2023-3434
- Pozios I, Vouliotis AI, Dilaveris P, Tsioufis C. Electro-mechanical alterations in atrial fibrillation: Structural, electrical, and functional correlates. J Cardiovasc Dev Dis. 2023;10(4):149. doi: 10.3390/jcdd10040149
- Raisi-Estabragh Z, McCracken C, Condurache D, et al. Left atrial structure and function are associated with cardiovascular outcomes independent of left ventricular measures: A UK Biobank CMR study. Eur Heart J Cardiovasc Imaging. 2022;23(9):1191–1200. doi: 10.1093/ehjci/jeab266
- Floria M, Radu S, Gosav EM, et al. Left atrial structural remodelling in non-valvular atrial fibrillation: What have we learnt from CMR? Diagnostics (Basel). 2020;10(3):137. doi: 10.3390/diagnostics10030137
- Kim HD, Cho DH, Kim MN, et al. Left atrial dysfunction, fibrosis and the risk of thromboembolism in patients with paroxysmal and persistent atrial fibrillation. Int J Heart Fail. 2022;4(1):42–53. doi: 10.36628/ijhf.2021.0043
- Schönbauer R, Kammerlander AA, Duca F, et al. Prognostic impact of left atrial function in heart failure with preserved ejection fraction in sinus rhythm vs persistent atrial fibrillation. ESC Heart Fail. 2022;9(1):465–475. doi: 10.1002/ehf2.13723
- Chistyakova MV, Govorin AV, Mudrov VA, et al. Heart damage and endothelial dysfunction in patients with coronavirus infection. Therapists Bulletin. 2023;(1):1–7. (In Russ).
- Rienstra M, van Veldhuisen DJ, Hagens VE, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation. J Am Coll Cardiol. 2005;46(7):1298–306. doi: 10.1016/j.jacc.2005.05.078
- Proietti M, Raparelli V, Basili S, et al. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: The AFFIRM Trial. Int J Cardiol. 2016;(207):258–263. doi: 10.1016/j.ijcard.2016.01.169
- Yaroslavskaya EI, Krinochkin DV, Shirokov NE, et al. Clinical and echocardiographic profile of patients one year after COVID-19 pneumonia depending on the left ventricular global longitudinal strain. Siberian J Clin Experimental Med. 2022;37(4):52–62. (In Russ). doi: 10.29001/2073-8552-2022-37-4-52-62
- Wong GR, Nalliah CJ, Lee G, et al. Sex-Related differences in atrial remodeling in patients with atrial fibrillation: Relationship to ablation outcomes. Circ Arrhythm Electrophysiol. 2022;15(1):e009925. doi: 10.1161/CIRCEP.121.009925
- Bräuninger H, Stoffers B, Fitzek AD, et al. Cardiac SARS-CoV-2 infection is associated with pro-inflammatory transcriptomic alterations within the heart. Cardiovasc Res. 2022;118(2):542–555. doi: 10.1093/cvr/cvab322
- Wu L, Jiang Z, Meulendijks ER, et al. Atrial inflammation and microvascular thrombogenicity are increased in deceased COVID-19 patients. Cardiovasc Pathol. 2023;(64):107524. doi: 10.1016/j.carpath.2023.107524
Supplementary files