Quality management system: A tool for the development of the organization or an additional burden?
- Authors: Zayunchkovskiy S.Y.1, Konovalov S.A.1, Zinchenko V.V.1, Sharova D.E.1, Ahkmad E.S.1, Vladzymyrskyy A.V.1
-
Affiliations:
- Moscow Center for Diagnostics and Telemedicine
- Issue: Vol 4, No 3 (2023)
- Pages: 439-447
- Section: Correspondence
- Submitted: 27.06.2023
- Accepted: 06.07.2023
- Published: 26.09.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/514629
- DOI: https://doi.org/10.17816/DD514629
- ID: 514629
Cite item
Abstract
A quality management system constitutes one of the organization’s management systems that provides for the selection of a set of processes in the organization’s activities designed to ensure the stable quality of products and services provided.
The growth of global industrial production has underscored the need for the creation of such production and management systems. These systems are designed to ensure that enterprises remains prepared to meet the constantly changing consumer value of manufactured products in accordance with consumer requirements, as well as the satisfaction of consumers themselves. As a result, attention began to focus on the production processes implemented within the organization when creating products. Regarding the production of medical devices, a quality management system can be defined as an organizational structure encompassing its functions, procedures, processes, and resources necessary for the coordinated direction and management of a manufacturing organization with respect to the quality of medical products.
The article reflects the principles of the quality management system and management processes. Noteworthy emphasis is placed on the features of quality management systems for medical devices, including the features of the quality management system for software that is a medical device. Furthermore, the conditions under which the quality management system becomes a tool for ensuring the sustainable development of the organization are noted.
Full Text
INTRODUCTION
General applicability of quality management system principles
A quality management system (QMS) is one of the organization’s management systems. A QMS identifies organizations’ processes to ensure the consistent quality of products and services. A QMS is designed to improve these processes as a set of interrelated and interacting activities and develop a management style for an organization that involves managers, engineers, and technical as well as support personnel in improving product quality. Following the implementation of a QMS changes aimed to provide technological transparency of all types of activities. Such standards enable tracking of a product’s whole life cycle in which the organization is involved, from the decision to develop a product to its final stage of disposal. Such an approach helps systematize an organization’s activities, create conditions for the self-fulfillment of process participants, improve product quality, and become more competitive.
According to ISO 9001,1 the scheme of QMS development is universal for any organization; hence, it is important to highlight special aspects of its activities and properly divide them into key processes.
Basic processes of a Russian organization
For a long time, corporate management in Russia was exclusively “functional.” Such an approach was quite viable. It was mainly limited to delegating responsibility for individual functions in specific activity areas (design, production, supply, sales, business and infrastructure maintenance, and after-sales service) to the corresponding functions as well as their managers and employees. Simultaneously, the aim of such functional management (heads of services, departments, and higher-level units) was to ensure compliance of specific (highly specialized) functional activities with internal quality criteria, which were established by corporate standards [1]. However, the functional management approach failed as global industrial production developed. This required the development of production and management systems capable of ensuring both customer satisfaction through providing the highest consumer values and the organization’s capacity to be prepared for constantly changing consumer value of their products in compliance with consumer requirements. Processes related to product development and manufacturing received special attention.
A QMS in the manufacturing of medical devices is defined as the organizational structure, functions, procedures, processes, and resources required for coordinated activities to manage the quality of healthcare products manufactured by an organization. A medical device QMS should ensure compliance of released medical devices with relevant general safety and effectiveness criteria, labeling, and technical as well as operational documentation requirements. The need to implement and comply with a QMS is not absolute in healthcare; not everyone must implement and maintain a QMS for healthcare products, but everyone has the right to do so.
A MEDICAL DEVICE QMS
A new trend is emerging to ensure the quality and safety of medical devices at all stages of their life cycle. Medical device regulators are shifting their focus from product design and development to the whole life cycle of a medical device, including steps from production to decommissioning. This approach is reflected in ISO 13485.2
Effective medical device life cycle management is crucial for ensuring end-user safety [2]. Therefore, in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council on Medical Devices [3], CE certification of medical devices is based on an assessment of procedures regulating product development and manufacturing. At one stage of medical device CE certification, the QMS should be confirmed to conform with ISO 13485.
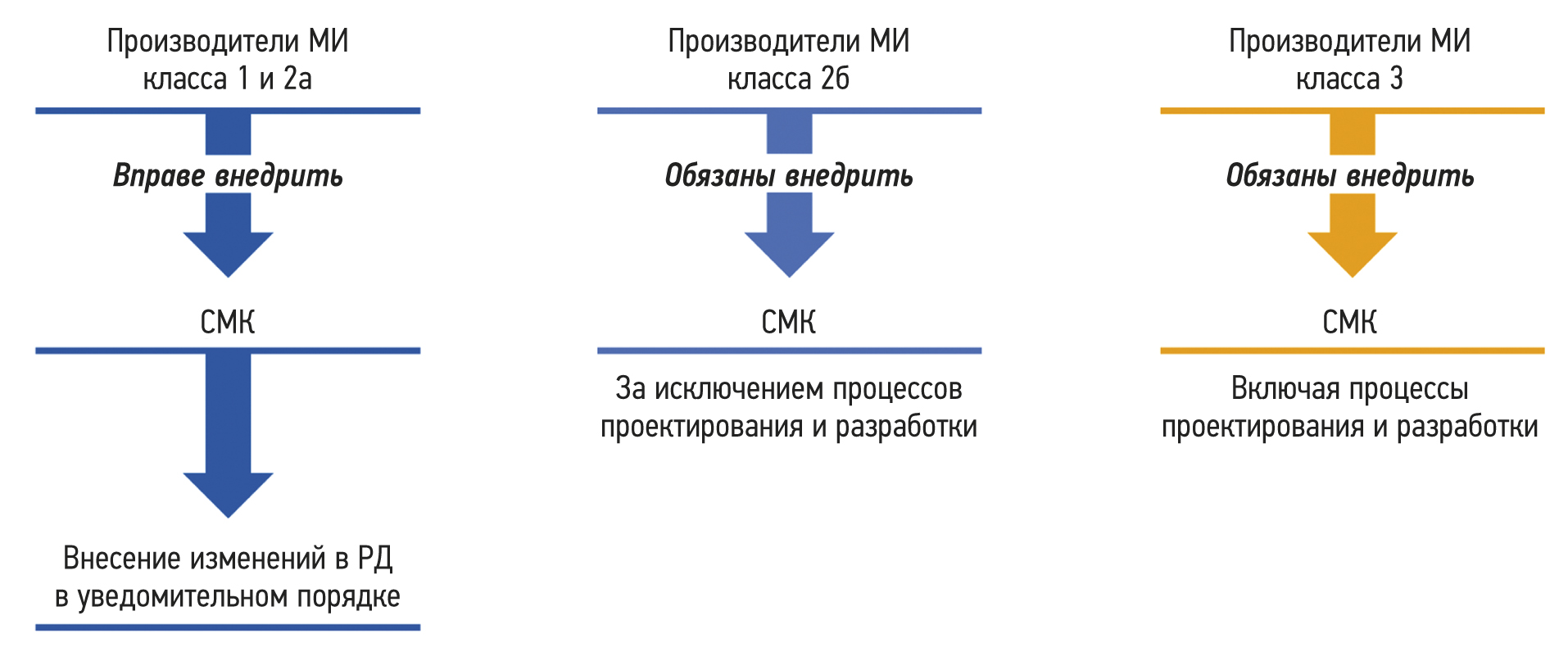
Manufacturers of medical devices (except for Class 1 medical devices and nonsterile Class 2a medical devices due to potential harm to users) must implement a QMS depending on medical device class3, 4 before submitting approval documents as part of medical device regulation in the Eurasian Economic Union. Figure 1 demonstrates a scheme of requirements for medical device QMS implementation based on potential harm to users. Suppose a manufacturer of medical devices has implemented a QMS in accordance with standards equivalent to ISO 13485. In that case, the QMS’s compliance with such standards should be confirmed (certificate of compliance and audit report) to ensure compliance with Decision No. 106 of the Council of the Eurasian Economic Commission dated November 10, 2017, regarding processes and procedures for using a medical device QMS .5
Fig. 1. Requirements for implementing a medical device quality management system depend on the potential risk of their use. GD, governing document; MD, medical devices; QMS, quality management system.
Special aspects of a medical device QMS
Special aspects of a medical device QMS are related to some requirements for products (medical devices) and their properties associated with such requirements. These properties should be distinct or require special attention compared with other types of industrial products. They are included in basic documents that govern the design, development, and distribution of medical devices. Medical device requirements include safety, effectiveness, and quality. A medical device QMS should ensure compliance with these requirements. In general, an organization must meet the following criteria (in accordance with ISO 13485):
- to identify current risks and risk situations
- to perform risk monitoring/analysis and explore parameters regularly
- to provide internal control of an organization’s activities
- to ensure necessary adjustments to be made
- to improve a medical device QMS, including its components, and
- to develop supporting documentation and comply with other conditions specified in ISO 13485.
A medical device QMS should be designed to encourage compliance with the key requirement to ensure that the benefit outweighs possible adverse effects. Medical devices should be safe. Medical device effectiveness is another important QMS function. If the effectiveness of a medical device is not mandatory, there is no point in developing and manufacturing such a product. Quality is an umbrella term for a set of such properties and characteristics of a medical device that affect its ability to function as intended in accordance with the manufacturer’s requirements.
Special attention should be given to QMS processes for evaluating the quality, effectiveness, and safety of medical devices, primarily performed as part of state registration, a procedure developed to ensure product market circulation. The deliverable is a marketing authorization, a document confirming medical device compliance with established requirements and medical device approval in Russia. A marketing authorization is mandatory for medical device circulation on the market. Therefore, state registration processes should be included in the overall QMS of an organization manufacturing medical devices. This is another important aspect of a Russian organization’s QMS.
To summarize, a medical device QMS is primarily related to life cycle processes, and medical device circulation is an integral part of these processes.
Special aspects of medical device software QMS
Regarding medical device software, some additional aspects of a QMS that are relevant to the manufacturing process may be highlighted. In contrast to physical products, software manufacturing does not require assembly and manufacturing sites. However, manufacturing medical device software requires strict quality and safety control at all stages of the product life cycle.
Software, unlike physical products, may be constantly updated, requiring quality management, including design, performance, and risk reviews. Otherwise, product failure may lead to incorrect conclusions, potentially harming patient health. To prevent such situations, medical device software should be controlled at the design, development, risk management, and manufacturing stages. Corrective and preventive actions should be implemented in accordance with the QMS.
Moreover, security and privacy risks should be managed because many software systems rely on communication technologies and are vulnerable to cyber-attacks that could lead to failures or leaks of patient information. The Korea Ministry of Food and Drug Safety has published application methods, cybersecurity cases, and cybersecurity risk management guidelines [4].
A software QMS should include design, development, testing, verification and validation, documentation, and personnel training. A QMS should be in place to ensure the software’s safety, reliability, and performance. To ensure the safety and performance of their software, healthcare organizations must adhere to all aspects of their QMS.
Special characteristics of an artificial intelligence–based medical device software QMS: National standard
In general, an artificial intelligence (AI)–based medical device software QMS should adhere to ISO 13485 while considering special aspects of using AI technologies in healthcare.
Requirements for the development, testing, and operation of AI-based healthcare technologies should be unified and standardized by the Artificial Intelligence in Healthcare subcommittee of the Technical Artificial Intelligence Standards Committee (PK 01/ТК 164). PK 01/ТК 164 is based on the Center for Diagnostics and Telemedicine Technologies of the Moscow Healthcare Department [5]. As part of the PK 01/ТК 164 activities, a series of national AI Systems in Clinical Medicine standards was developed.
GOST R 59921.8-2022 is one of these standards.6 The document includes recommendations for interpreting all sections of GOST ISO 13485-2017, as well as cases, descriptions, and options that may be used by organizations in developing and implementing a QMS in compliance with these standards.
Therefore, when developing and implementing a QMS, manufacturers of AI technologies should consider the above aspects. Industry QMS requirements have changed from ISO 9001 to GOST R 59921.8; some requirements about special aspects of medical devices have been added or clarified, and guidelines for applying ISO 13485 to processes of ordering, supply, development, use, and maintenance of AI systems have been developed (Figure 2).
Fig. 2. Development of industry requirements for an AI-based medical device quality management system.
An AI-based software QMS and its impact on the competitiveness and potential of an organization
A QMS for AI technology directly impacts an organization’s competitiveness and potential. Users will be more confident in a product, promoting an organization on the market if tasks are performed correctly, the safety and effectiveness of AI technologies are provided, and their risks are managed.
For this paper, a brief survey of AI technology manufacturers was conducted. Respondents were asked to answer questions on implementing a QMS that complied with ISO 13485. The list of questions is presented in Table 1. The survey included 10 AI technology manufacturers. The number of employees in the organizations surveyed ranged from 10 to 600 people. When asked about the QMS implementation, 60% of respondents answered positively. Of the remaining 40% of companies, 75% planned to develop and implement a QMS. Changes in business processes after QMS implementation were rated 3 to 9 points on a 10-point scale. It should be noted that the QMS implementation may encounter resistance from the part of the team; therefore, at the stage of QMS implementation, it is necessary to ensure that each team member understands the significance of this process. The level of team resistance to QMS implementation was 40%. All surveyed organizations with certified ISO 13485 QMS compliance used the services of a consulting company for QMS development as well as implementation and appointed a person responsible for QMS. When developing and implementing a QMS, 33% of surveyed companies used the national standard GOST R 59921.8-2022.
Table 1. Survey of AI technology manufacturers on the availability, use, and implementation of a quality management system
No. | Question |
1 | Number of employees |
2 | Has your organization implemented a quality management system (QMS)? |
3 | If not, do you plan to develop and implement a QMS? |
4 | Do you have a certificate of QMS compliance with ISO 13485:2016 or GOST ISO 13485-2017? |
5 | Which certification system was used to obtain a certificate? |
6 | How much have your business processes changed after the QMS implementation? Rate on a scale of 0 to 10. |
7 | Rate your team’s resistance to the QMS implementation on a scale of 0 to 10. |
8 | Did you use the services of a consulting company when developing and implementing a QMS? |
9 | Do you have a person responsible for QMS? |
10 | Did you use national standard GOST R 59921.8-2022 Artificial Intelligence Systems in Clinical Medicine? Part 8. Guidelines for application of GOST ISO 13485-2017 when developing and implementing a QMS? |
11 | Does your QMS comply with Decree No. 136 of the Government of the Russian Federation, dated February 9, 2022, on approval of requirements for implementing, maintaining, and evaluating the medical device QMS, depending on the potential risk of their use? |
12 | Does your QMS comply with Decision No. 106 of the Council of the Eurasian Economic Commission dated November 10, 2017, on approval of requirements for implementation, maintenance, and evaluation of a medical device quality management system depending on potential harm to users? |
CONCLUSION
The main objective of a medical device QMS is to provide benefits without creating additional burdens on the organization during the life cycle. However, it is challenging to integrate a medical device QMS into the ongoing activities of an organization, especially if a medical device QMS is not mandatory. In this case, stimulating factors for ISO 13485 certification may include an organization’s higher status as a tender participant, wider opportunities for supplying products outside of the Russian Federation, increased trust of end users, and improved internal business processes. A decision to integrate a medical device QMS should not be formal to simply obtain a certificate of compliance but instead, be driven by an organization’s conscious internal requirements to use a medical device QMS as a sustainability tool.
ADDITIONAL INFORMATION
Funding source. This article was prepared by a group of authors as a part of the research and development effort titled “Theoretical and methodological framework for digital transformation in radiology” (USIS No. 123031400118-0) in accordance with the Order No. 1196 by the Moscow Health Care Department dated December 21, 2022 “On approval of state assignments funded by means of allocations from the budget of the city of Moscow to the state budgetary (autonomous) institutions subordinate to the Moscow Health Care Department, for 2023 and the planned period of 2024 and 2025”.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. S.Yu. Zayunchkovsky ― writing the manuscript, structuring and analyzing the obtained results, a bibliography; S.A. Konovalov ― writing the manuscript, analyzing the survey results; V.V. Zinchenko ― structuring and analyzing the obtained results, writing the manuscript; D.E. Sharova ― generating a research hypothesis, developing a questionnaire; E.S. Ahmad ― analyzing the obtained results, reviewing the manuscript; A.V. Vladzimirsky ― reviewing the manuscript, the overall guidance.
1 ISO 9001:2015 Quality management systems ― ―Requirements. This standard was last reviewed and confirmed in 2021. Therefore, this version remains valid. Link: https://www.iso.org/standard/62085.html.
2 ISO 13485:2016 Medical devices. Quality management systems. Requirements for regulatory purposes. This standard was last reviewed and confirmed in 2020. Therefore this version remains current. Link: https://www.iso.org/standard/59752.html.
3 Decree N 136 of the Government of the Russian Federation dated February 9, 2022 on approval of requirements for the implementation, maintenance and evaluation of the medical device quality management system depending on potential risk of their use. Link: https://base.garant.ru/403517950/.
4 Decision No. 106 of the Council of the Eurasian Economic Commission dated November 10, 2017 on approval of requirements for implementation, maintenance and evaluation of a medical device quality management system depending on potential harm to users. Link: https://pharmvestnik.ru/documents/reshenie-soveta-evrazijskoj-ekonomicheskoj-komissii-ot-10-11-2017-g-106.html.
5 Ibid.
6 ГОСТ Р 59921.8-2022. Artificial Intelligence Systems in Clinical Medicine: national standard of the Russian Federation. Part 8. Guidelines for the application of GOST ISO 13485-2017 Link: https://docs.cntd.ru/document/1200193729.
About the authors
Sergey Yu. Zayunchkovskiy
Moscow Center for Diagnostics and Telemedicine
Email: ZayunchkovskijSY@zdrav.mos.ru
ORCID iD: 0009-0002-7463-7699
Russian Federation, Moscow
Sergey A. Konovalov
Moscow Center for Diagnostics and Telemedicine
Email: KonovalovSA4@zdrav.mos.ru
ORCID iD: 0009-0003-0011-3371
Russian Federation, Moscow
Viktoria V. Zinchenko
Moscow Center for Diagnostics and Telemedicine
Author for correspondence.
Email: ZinchenkoVV1@zdrav.mos.ru
ORCID iD: 0000-0002-2307-725X
SPIN-code: 4188-0635
Russian Federation, Moscow
Daria E. Sharova
Moscow Center for Diagnostics and Telemedicine
Email: SharovaDE@zdrav.mos.ru
ORCID iD: 0000-0001-5792-3912
SPIN-code: 1811-7595
Russian Federation, Moscow
Ekaterina S. Ahkmad
Moscow Center for Diagnostics and Telemedicine
Email: AkhmadES@zdrav.mos.ru
ORCID iD: 0000-0002-8235-9361
SPIN-code: 5891-4384
Russian Federation, Moscow
Anton V. Vladzymyrskyy
Moscow Center for Diagnostics and Telemedicine
Email: VladzimirskijAV@zdrav.mos.ru
ORCID iD: 0000-0002-2990-7736
SPIN-code: 3602-7120
MD, Dr. Sci. (Med.)
Russian Federation, MoscowReferences
- Mikhailov YuI. Process management in the quality management system of the enterprise. Discourse. 2017;(6):51–57. (In Russ).
- Zinovieva EV, Sapunova AV, Ivanov IV. Safety of circulation of medical devices at all stages of their life cycle. Public health. 2022;2(3):16–24. (In Russ). doi: l0.21045/2782-1676-202l-2-3-16-24
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC // Official J Eur Union. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745. Accessed: 15.07.2023.
- Lim K, Heo TY, Yun J. Trends in the approval and quality management of artificial intelligence medical devices in the Republic of Korea. Diagnostics (Basel). 2022;12(2):355. doi: 10.3390/diagnostics12020355
- Gusev AV, Vladzimirsky AV, Sharova DE, et al. Development of research and development in the field of artificial intelligence technologies for healthcare in the Russian Federation: Results of 2021. Digital Diagnostics. 2022;3(3):178–194. (In Russ). doi: 10.17816/DD107367
Supplementary files