Possibilities and limitations of magnetic resonance imaging in the diagnostics of endocervical adenocarcinomas
- Authors: Antonova I.B.1, Aksenova S.P.1, Nudnov N.V.1,2,3, Kriger A.V.1
-
Affiliations:
- Russian Scientific Center of Roentgenoradiology
- Peoples’ Friendship University of Russia
- Russian Medical Academy of Continuous Professional Education
- Issue: Vol 5, No 2 (2024)
- Pages: 149-166
- Section: Original Study Articles
- Submitted: 19.09.2023
- Accepted: 04.12.2023
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/585195
- DOI: https://doi.org/10.17816/DD585195
- ID: 585195
Cite item
Abstract
BACKGROUND: In recent decades, the incidence of cervical adenocarcinomas has increased from 5% to 20%. Endocervical adenocarcinomas are characterized by a more aggressive course and early metastasis. Owing to the difficulties in the cytological diagnosis of cervical adenocarcinoma, early radiation diagnostics and staging subsequently play a key role. Very few studies have examined the use of magnetic resonance imaging in diagnosing cervical adenocarcinomas.
AIM: To determine the diagnostic informativeness of magnetic resonance imaging in the staging of cervical adenocarcinomas according to the T-criterion and assessing the depth of tumor invasion into the stroma of the cervix and clarify the semiotic signs of adenocarcinoma and features of tumor growth in the uterus.
MATERIALS AND METHODS: In total, 123 patients diagnosed with cervical cancer (C53), who underwent diagnosis and treatment between 2020 and 2023, were examined. The examination results of 22 (18%) patients with cervical adenocarcinoma were analyzed. The average patient age was 56 years. A multiparametric magnetic resonance examination of the pelvic organs was performed on 22 patients using tomographs with a magnetic field strength of 1.5 T. Moreover, 14 (64%) patients underwent surgery including extirpation of the uterus and appendages with pelvic lymphadenectomy. The information value of magnetic resonance imaging was evaluated in 11 patients, whose first stage was surgical treatment.
RESULTS: In this study, cervical adenocarcinoma was detected in 18% among all cases of cervical cancer. The information value of magnetic resonance imaging in assessing the local prevalence of endocervical adenocarcinoma according to the T-criterion was as follows (main value with the corresponding 95% confidence interval): sensitivity, 77.78% (39.99%–97.19%); specificity, 50.00% (1.26%–98.74%); positive predictive value, 87.50% (62.64%–96.69%); negative predictive value, 33.33% (7.30%–76.04%); and accuracy, 72.73% (39.03%–93.98%). The information value of magnetic resonance imaging in assessing the depth of tumor invasion into the cervical stroma was as follows: odds ratio, 3.500 (0.145%–84.694%); sensitivity, 85.7% (0.757%–0.993%); specificity, 33.3% (0.018%–0.0648%); positive predictive value, 75% (0.673%–0.883%); negative predictive value, 50% (0.027%–0.972%).
CONCLUSIONS: The results of this study showed that magnetic resonance imaging is a good tool with high diagnostic informativeness in detecting endocervical cervical adenocarcinoma. The four macrostructures of tumor growth in endocervical adenocarcinoma identified during magnetic resonance imaging data analysis indicate locally aggressive tumor growth and a high frequency of endometrial dropouts. This finding will allow radiologists to structure a descriptive picture, including the verified cervical adenocarcinoma, to enhance methods of developing a treatment plan for the patient.
Full Text
BACKGROUND
Despite widespread adoption of preventive measures, cytology screening, and active treatment for precancerous conditions, the incidence of cervical cancer (CC) remains high. According to the World Health Organization, CC is the fourth most common cancer in women worldwide in terms of prevalence and mortality [1]. In developed countries, the incidence rate tends to decrease [1, 2]. In Russia, CC is consistently the fifth common cause of cancer morbidity and the tenth common cause of cancer mortality. Additionally, most de novo cases occur in women of reproductive age (40–49 years). Over the past decade, the CC population has increased by 10% [3].
In 2011, a meta-analysis showed that the increased number of CC cases was associated with human papillomavirus (HPV) types 16, 18, and 31 and other types. In cervical squamous cell carcinoma, which is associated with HPV in up to 90% of cases, type 16 was the most common (59.3%) [4]. Cervical adenocarcinoma (CA) is the second most common histologic variant of CC, with 75% of cases associated with HPV; either type 18 or 16 predominate, depending on the country. HPV type 18 was detected in 36.8% of all HPV-positive adenocarcinomas [5, 6]. The incidence of other histologic types of cancer does not exceed 1% [7, 8]. CA is characterized by significant histologic heterogeneity. The association of CA with HPV served as the basis for a new pathogenetic classification published in 2018 (IECC, International Endocervical Adenocarcinoma Criteria and Classification). HPV-positive adenocarcinomas are classified as usual, villoglandular, mucinous, intestinal, and signet ring cell adenocarcinomas and HPV-associated adenocarcinoma not otherwise specified. HPV-negative CAs, which account for 15%–20%, include gastric, clear cell, mesonephric, serous, and endometrioid subtypes and adenocarcinoma not otherwise specified [9, 10].
The classification of HPV-positive and HPV-negative endocervical adenocarcinomas is based on clinical features, differences in tumor biology, prognosis, and response to treatment. The most common HPV-associated endocervical adenocarcinoma is the most typical subtype, accounting for ~75% of all endocervical adenocarcinomas [10]. Mucinous endocervical adenocarcinomas account for ~10% of all HPV-associated endocervical adenocarcinomas [9, 11]. Additionally, endometrioid endocervical adenocarcinoma is rare. With strict diagnostic criteria, it accounts for <1% of all CAs and is associated with endometriosis [9, 11].
CC screening and prevention reduces morbidity and mortality from invasive cervical squamous cell carcinoma. However, in recent decades, CA incidence has increased from 5% to 20% [4, 10, 12]. In addition to diagnostic issues, CA is characterized by a more aggressive course, early metastasis, and lower sensitivity to radiation and drug therapy and represents a serious problem in gynecologic oncology practice [13–15]. The specific location and course of CA with obvious diagnostic problems lead to late detection of the tumor and a high frequency of recurrence and mortality [4, 12]. Therefore, improving diagnostics and developing early detection algorithms are crucial for successful treatment.
According to the 2019 International Federation of Gynecology and Obstetrics (FIGO) classification, stages IA2, IB1, and IIA1 are considered local CC forms; stages IB2 and IIA2–IVA are considered locally advanced; and tumors with distant metastases are classified as advanced stage IVB tumors [16]. Clinical staging of CC (including CA), particularly according to the revised 2018 FIGO classification, is based on a comprehensive evaluation, which includes history-taking and physical examination, morphologic review, and imaging data, such as magnetic resonance imaging (MRI), ultrasound (US), computed tomography (CT), and positron emission tomography (PET). Accuracy of clinical staging at initial diagnosis is critical to the success of subsequent treatment and prognosis [7, 16]. Staging of local CC is based on tumor size; however, the size of the primary cervical lesion does not define the stage of locally advanced tumor when the vagina, parametrium, ureters, and adjacent organs are involved. Although in CC staging, the size of the primary tumor is considered, the recent FIGO report indicated that cervical stromal invasion of >50% and involvement of the outer parts of the stromal ring are associated with poor prognosis and increased recurrence rate [16].
CA does not develop from the squamocolumnar junction (as in cervical squamous cell carcinoma), but from the deep part of the cervical canal, within the crypts; this defines the type of tumor growth, which is predominantly endophytic, resulting in the absence of early clinical manifestations and late detection of the tumor. Tumor progression in the deep endocervix, closer to the internal orifice of the cervix, leads to tumor spread in the adjacent isthmus and endometrium, infiltrating the cervical stroma and myometrium, mimicking endometrial cancer (EC). When tumor imaging (using US, MRI, and CT) is performed at the stage of simultaneous uterine body and cervical involvement, determination of the primary lesion and staging become extremely difficult. According to the FIGO classification, in CC, tumor spread to the body of the uterus does not affect the stage, whereas in EC with cervical involvement, the tumor progresses from stage T1 to T2, which significantly affects the treatment choice and prognosis. In these cases, histology and immunohistochemistry are the definitive diagnostic tests. Immunohistochemical markers for differential diagnosis include p16, estrogen and progesterone receptors, and p53 [17]. Positive staining for p16 is more characteristic for usual-type HPV-associated endocervical adenocarcinoma, and a positive test for estrogen and/or progesterone receptors is more common in endometrioid adenocarcinoma of the endometrium, although it may also be seen in CA [17]. In 2022, a Korean research group led by Song JY trained artificial intelligence to differentiate between different subtypes of cervical and uterine body cancer. The study demonstrated high diagnostic efficacy of the proposed algorithm (AUC of 0.977 for CC, 0.944 for EC, and 0.939 for differentiation of cervical and uterine body adenocarcinoma) [18].
A 2020 meta-analysis evaluated the diagnostic efficacy of various imaging modalities (i.e., MRI, US, CT, and PET) in assessing local tumor spread and lymph node metastases in patients with newly diagnosed CC and showed that MRI had the highest sensitivity and specificity for local spread of CC. All the above modalities have a high specificity for detecting metastases in lymph nodes [19]. In some studies, including Russian studies (Rubtsova NA et al.), the overall accuracy of MRI in staging invasive CC was 77%–90% [20, 21]. MRI provides high resolution of soft tissues and more accurately determines the depth of invasion and preoperative tumor size, especially with T2-weighted images (WI). However, the diagnostic value of MRI in assessing parametrial invasion remains controversial, with a borderline sensitivity rate (~73%–76%), associated with a high percentage of false-positive results [22, 23]. Studies that evaluated the characteristics of MRI in endocervical adenocarcinoma are few.
STUDY AIM
To determine the diagnostic efficacy of MRI in T-staging CC and in assessing the depth of cervical stromal invasion of endocervical adenocarcinoma and identify semiotic signs of adenocarcinoma and characteristics of uterine tumor growth.
MATERIALS AND METHODS
Study Materials. Study Design
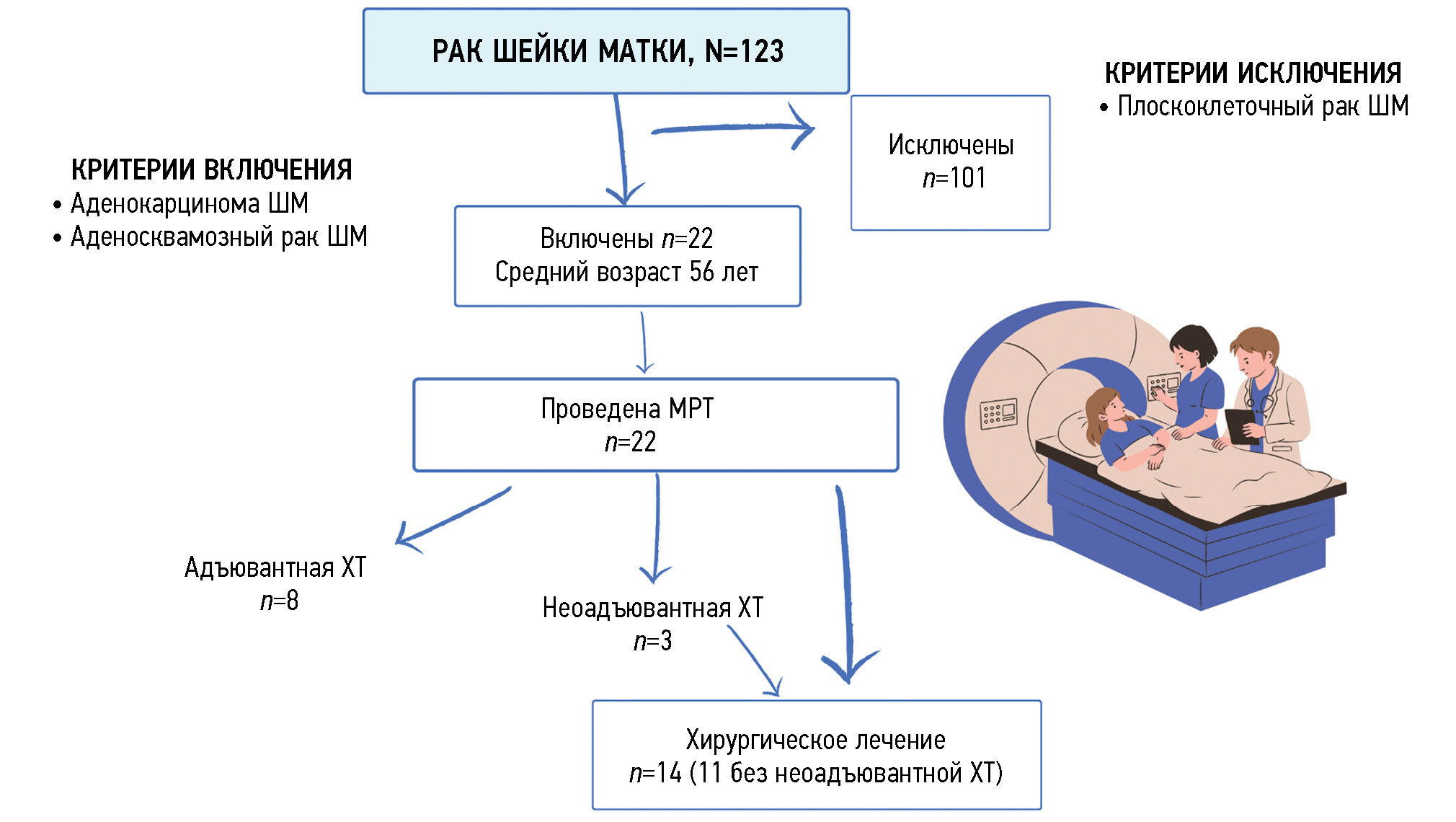
This single-center, retrospective, selective study included 123 patients with histologically confirmed CC (ICD code: C53) to evaluate the incidence trend of CA. They were examined and treated at the Scientific Center for Radiography and Radiology of the Ministry of Health of the Russian Federation from 2020 to 2023.
Patients with CA were comprehensively evaluated; the results of 22 (18%) patients with this histologic cancer type were reviewed. The mean age of patients with CA was 56 years (range: 35–74 years). Data analysis excluded patients with squamous cell carcinoma.
Fig. 1 shows the study design. Table 1 presents the distribution of patients by histologic tumor type and grade of differentiation. The distribution by FIGO stages is presented in Table 2.
Fig. 1. Study design. MRI, magnetic resonance imaging; CT, computed tomography.
Table 1. Distribution of patients depending on the histological type of tumor and degree of differentiation
Histologic type | No. of patients (n=22) | |
Endocervical adenocarcinoma | Low-grade | 7 |
Intermediate-grade | 4 | |
High-grade | 2 | |
Serous adenocarcinoma | 3 | |
Endometrioid adenocarcinoma | 5 | |
Adenosquamous carcinoma | 1 | |
Table 2. Distribution of patients by disease according to the International Federation of Gynecology and Obstetrics classification
Stage | Number of patients (n=22) |
Cancer in situ | 2 |
IB | 4 |
IB1 | 3 |
IB2 | 1 |
IIA | 1 |
IIB | 2 |
IIIB | 1 |
IIIC | 1 |
IIIC1 | 3 |
IV | 2 |
IVB | 2 |
Study Methods
Overall, 123 women were examined by an obstetrician-gynecologist. The study included assessment of symptoms and medical history, bimanual rectovaginal examination, vaginal and cervical speculum examination, cytology of cervical and endocervical smears, and cervical histology.
In 22 patients (18%), multiparametric pelvic MRI was performed using 1.5 T scanners. It was conducted using a flexible body coil and with the patient in supine position. The multiparametric MRI protocol included T1-WI and T2-WI; STIR; diffusion-weighted images with b-factors of 0, 800, and 1000 sec/mm2; and dynamic contrast enhancement with gadolinium salts, which met the European Society of Urogenital Radiology (ESUR) requirements for MRI [24]. According to ESUR guidelines, a b-factor of 1000 sec/mm2 is sufficient for the diagnosis of uterine disorders in routine practice.
T2-WI MRI data were used to evaluate the size and depth of cervical stromal invasion (Fig. 2); the presence of parametrial invasion; involvement of the internal orifice and isthmus of the uterus, endometrial lining, uterine appendages, and lymph nodes; and the presence of a “feeding pedicle” in the tumor. The tumor pedicle was used to designate the junction between the tumor and uterine wall, considered as the tumor origin, with tumor feeding vessels visualized in the arterial and venous phases of dynamic contrast enhancement.
Fig. 2. An example of measuring the depth of cervical adenocarcinoma invasion into the stroma and the distance from the tumor to the exocervix: (а) the tumor is located in the upper third of the cervix, has a depth of invasion of 8 mm and is located at a distance of 20 mm from the exocervix; (b) the tumor is located in the upper third and middle third of the cervix, has a depth of invasion of 6 mm and is located at a distance of 16 mm from the exocervix. The tumor is outlined with a purple line, the endocervical canal is marked with pink lines. Conclusion of the pathomorphological study: endocervical adenocarcinoma of the cervix grade 2; depth of invasion into the cervical stroma 5 mm (less than 1/2 the thickness of the cervical wall); lymphovascular invasion was detected; the tumor grows into the internal os; endometrium in the secretion phase.
Extirpation of the uterus and appendages with pelvic lymphadenectomy was performed in 14 patients (64%). Three patients (21%) underwent surgery after neoadjuvant polychemotherapy. Pathologists assessed the size and depth of cervical stromal invasion in 11 patients with CA who started anticancer treatment at the surgical stage. In the excised specimens, the presence of cervical stromal invasion, vaginal transition, parametrial invasion, uterine body involvement (depth of myometrial invasion), uterine appendages, and lymph nodes were also evaluated. In 11 patients, preoperative MRI data, obtained not more than 1 month prior to the start of the treatment, were compared with postoperative pathology data. Eight patients (36%) received a combination of chemotherapy and radiotherapy.
Microsoft Excel (Microsoft, USA) and JavaStat were used for statistical processing of the results.
Ethical Review
According to the Independent Ethics Committee at the Russian Scientific Center for Radiography and Radiology of the Ministry of Health of the Russian Federation (minutes of meeting no. 9, dated September 29, 2023), the study “Possibilities and Limitations of Magnetic Resonance Imaging in the Diagnostics of Endocervical Adenocarcinomas” did not require the opinion of the Independent Ethics Committee.
RESULTS
CA was detected in 18% (22/123) of CC patients examined between 2020 and 2023.
In 5 patients (23%), CC was discovered incidentally during a routine gynecologic examination. The disease manifested with spotting in 13 patients (59%), serous discharge in 2 patients (9%), dragging pain in lower abdomen in 6 patients (27%), and painful urination in 1 patient (5%).
Preexisting cervical conditions (ectropion, erosion, and chronic cervicitis) were noted in 8 patients (36%) and cervical dysplasia (grade 1–3) in 3 patients (14%). In 11 patients (50%), no cervical abnormalities were detected prior to the diagnosis of CA. Table 3 shows the gynecological examination data.
Table 3. Results of gynecological examination
Gynecological examination data | Yes n (%) | No n (%) |
Cervical lesion | 10 (45) | 12 (55) |
Parametrial lesion (clinically as thickened fornixes) | 11 (50) | 11 (50) |
Vaginal involvement | 11 (50) | 11 (50) |
In 2 women (9%), the MRI scan did not show a tumor. Table 4 shows the MRI data of 22 patients. The mean tumor volume in T2-WI was 25 cm3 (range: 1–71 cm3). The following results were obtained when evaluating the diagnostic efficacy of MRI in assessing the local extent of CA (T-staging) in 11 patients after surgery as the first treatment step:
- Sensitivity: 77.78% (95% confidence interval [CI]: 39.99%, 97.19%)
- Specificity: 50.00% (95% CI: 1.26%, 98.74%)
- Positive predictive value: 87.50% (95% CI: 62.64%, 96.69%)
- Negative predictive value: 33.33% (95% CI: 7.30%, 76.04%)
- Accuracy: 72.73% (95% CI: 39.03%, 93.98%)
Table 4. Magnetic resonance imaging data
Parameter | Yes n (%) | No n (%) |
Cervical stroma invasion | 17 (77) | 5 (23) |
Parametrial invasion | 9 (41) | 13 (59) |
Involvement of the internal cervix | 12 (55) | 10 (45) |
Involvement of the isthmus | 9 (41) | 13 (59) |
Involvement of the endometrium | 6 (27) | 16 (73) |
Involvement of the adnexa | 4 (18) | 18 (82) |
Involvement of lymph nodes | 9 (41) | 13 (59) |
Presence of a central feeding vessel | 9 (41) | 13 (59) |
In 8 patients (40%), the CC was located at a distance from the external orifice (mean: 11 mm; range: 4–18 mm). In this location, the tumors cannot be visualized during gynecologic examination because the external orifice is intact. Infiltration of the vaginal cervix was reported in 9 patients (45%). The mean apparent diffusion coefficient (ADC) of the tumor was 0.833 × 10−3 mm/sec (range: from 0.440 × 10-3 to 0.1282 × 10−3 mm/sec).
We identified two types of tumor growth in the cervical stroma: the most common were the endophytic type (75%, n = 15), characterized by diffuse enlargement and barrel-shaped cervical transformation (Fig. 3), and exophytic type, which occurred in 25% of cases in the present study (n = 5). Exophytic tumors were most commonly located in the vaginal cervix, with tumor masses prolapsing into the vagina or cervical canal lumen (Fig. 3). The exophytic tumor was represented by different histologic subtypes of CA:
- Poorly and moderately differentiated typical endocervical adenocarcinoma, 2 cases
- Poorly differentiated serous adenocarcinoma, 1 case
- Adenosquamous carcinoma, 1 case
- Endometrioid endocervical adenocarcinoma, 1 case
Fig. 3. Сervical adenocarcinoma growth pattern. The upper row of images is T2-weighted images in sagittal plane, the lower one — in axial plane.
Furthermore, endophytic tumors were characterized by different histologic types and differentiation patterns. No correlation was found for the type of tumor growth and its histologic subtype.
Differential diagnosis of the location of the primary tumor (whether the observed uterine abnormalities were endometrial carcinoma with cervical involvement or cervical carcinoma with endometrial involvement) was critical in the diagnostic search of gynecologists and radiologists for detecting uterine adenocarcinoma. All diagnostic controversies with predominant involvement in the uterine cavity were further reviewed by pathologists, and in all patients, endocervical adenocarcinoma (tumor of the cervix) was confirmed. Therefore, four types of tumor macrostructure were retrospectively classified based on the predominant location of the tumor according to MRI and pathology data (Fig. 4):
- Predominance of tumor in the cervix (n = 13; 65%)
- Predominance of tumor in the uterine body (n = 2; 10%)
- Equal involvement of endocervix and endometrium (n = 2; 10%)
- Isolated cervical lesion with CA lesion seeding into the uterine cavity (in the endometrium), confirmed by pathology and immunohistochemistry data (n = 3; 15%)
Fig. 4. Type of tumor macrostructure, T2-weighted images in sagittal plane, cervical adenocarcinoma.
Notably, type 2, 3, and 4 tumors were described as uterine body cancer on MRI, and only pathology examination confirmed primary CA.
According to pathology data, the mean depth of cervical stromal invasion of adenocarcinoma was 8.2 mm (range: 2–15 mm). Surgical material examination data are presented in Table 5.
Table 5. Surgical material data of 14 patients
Parameter | Yes n (%) | No n (%) |
Cervical stroma invasion | 13 (93) | 1 (7) |
Parametrial invasion | 0 (0) | 14 (100) |
Involvement of the endometrium | 4 (29) | 10 (71) |
Involvement of the adnexa | 2 (14) | 12 (86) |
Involvement of lymph nodes | 2 (14) | 12 (86) |
A comparative analysis of the invasion depth according to MRI and postoperative pathology was performed in 11 patients who did not receive neoadjuvant chemotherapy (Table 6). MRI data on cervical stromal invasion depth revealed a false-positive result (overdiagnosis) in 2 patients (18%) and a false-negative result (underdiagnosis) in 1 patient (9%). In 8 patients (73%), MRI and pathology data were consistent. The thickness of the MRI slice (T2-WI) was 4 mm; thus, a difference between MRI and pathology data ≤4 mm was considered a method error.
Table 6. Diagnostic value of magnetic resonance imaging in the diagnosis of cervical adenocarcinoma
Parameter | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
Cervical stroma invasion evaluation | 85.7% (CI, 0.757–0.993) | 33.3% (CI, 0.018–0.0648) | 75% (CI, 0.673–0.883) | 50% (CI, 0.027–0.972) |
T-staging | 77.78% (CI, 39.99%–97.19%) | 50.00% (CI, 1.26%–98.74%) | 87.50% (CI, 62.64%–96.69%) | 33.33% (CI, 7.30%–76.04%) |
Note. CI, 95% confidence interval.
Therefore, the diagnostic value of MRI in assessing the depth of cervical stromal invasion of CA was as follows:
- Odds ratio: 3.500 (95% CI: 0.145, 84.694)
- Sensitivity: 85.7% (95% CI: 0.757, 0.993)
- Specificity: 33.3% (95% CI: 0.018, 0.0648)
- Positive predictive value: 75% (95% CI: 0.673, 0.883)
- Negative predictive value: 50% (95% CI: 0.027, 0.972)
The locally invasive growth pattern with tumor pedicle formation was observed, and feeding vessels were visualized (41%) in the endometrial CA seeding lesions (Fig. 5).
Fig. 5. Magnetic resonance imaging of the pelvis (cervical adenocarcinoma), metastasis in endometrium:
(a) from left to right and from top to bottom: T2-weighted image, T1FS-weighted image with contrast enhancement in the arterial phase, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) map. The tumor (metastasis in endometrium) is marked with an arrow. The region of the “leg” of the tumor and feeding vessels are marked with a dotted arrow; (b) sagittal plane, T2-weighted image (left) and T1FS+C (right): primary tumor (arrow), metastasis in the body of the uterus (star), vessels in the focus of tumor (dotted arrow). In the arterial phase of DСE, the accumulation of paramagnetic by the basal layer of the endometrium and the vessels in the “feeding leg” of the tumor is determined; (c) histological examination of the surgical material, stained with hematoxylin and eosin, ×10; d, immunohistochemical study, expression of p16.
Conclusion of the pathomorphological study: Moderately differentiated endocervical adenocarcinoma. The tumor grows into the stroma of the cervix to a depth of 1.3 cm (2/3 of the thickness of the wall of the cervix in the transition zone). The tumor grows into the myometrium (to a depth of 0.6 cm, 1/3 of the thickness of the uterine body wall) and endometrium. The vaginal part of the cervix is covered with a multilayer flat epithelium.
DISCUSSION
In our study, 18% (22/123) of patients diagnosed with CC had adenocarcinoma and mixed adenosquamous lesions of the cervix. This confirms the relative increase in CA morbidity reported in global (Chan ZF et al. and Islami F et al.) and Russian (Grigoruk OG et al.) studies [4, 10, 12]. We believe that the relative increase in CA morbidity is related to the lack of signs of the disease (in our study, 48% of patients had no visual cervical lesions on gynecologic examination and had an intact external orifice on MRI) and the lack of effective screening strategies. A population study by Castanon A et al. showed that current cytology screening is ineffective in the diagnosis of cervical pre-cancer (adenocarcinoma in situ or low-grade cervical glandular intraepithelial neoplasia), but it is effective in the detection of the earlier stage CA (stage IA). This is attributed to the predominantly endocervical location of CA, within the cervical crypts, which complicates the sampling of material containing atypical cells [25]. Islami F et al. found the increased incidence of CA in situ and invasive CA, mainly in young women aged 35–54 years, owing to improved CC screening [12]. Similar data were obtained in studies by Chan ZF et al. and Suh DH et al. [4, 26].
Regarding precancerous conditions, gastric-type endocervical carcinoma is preceded by endocervical glandular hyperplasia, including atypical adenocarcinoma in situ [27, 28]. In the present study, 50% of the patients had preexisting cervical diseases (e.g., chronic cervicitis); atypical adenocarcinoma in situ was found in one patient with history of multiple cervical conization caused by severe dysplasia (CIN III). Ten patients had endometriosis.
In this study, despite the high diagnostic value of MRI in detecting and assessing the extent of CC, the tumor was not visualized with multiparametric scanning tomography in 2 patients (9%) with histologically confirmed CA. This may be due to the small volume of the tumor and its lateral-spreading growth along the cervical canal and the technical limitations of this imaging modality.
Good results were obtained when comparing MRI and pathology examination data:
- The overall accuracy of MRI in assessing the depth of cervical stromal invasion was 70%, with a sensitivity of 87.5%.
- In assessing tumor extent (T-staging), the accuracy was 72.3% and the sensitivity was 77.8%.
In our study, in the case of underdiagnosis (n = 1), differences were associated with tumor necrosis leading to magnetic resonance signal heterogeneity in T2-WI and not accounted for in size measurement. In cases of overdiagnosis (n = 2), difference was due to severe uterine deformity in mixed adenomyosis and submucosal leiomyomas.
Despite the several advantages of MRI, foreign literature showed frequent discrepancies between clinical staging and surgical findings, with a tendency to underestimate stage; the higher the stage, the greater the discrepancy. For CA, these differences were even more significant [29, 30].
In addition to objective errors in assessing the extent of the various histologic forms of CC, overdiagnosis results from the concomitant inflammatory infiltration following invasive cervical manipulation or necrosis of large tumors. Underdiagnosis may result from the accumulation of retention cysts in the vaginal cervix, including the area around the external orifice, which complicates assessment of the structure of the epithelial lining and underlying cervical stroma [31].
For CA seeding in the endometrium, a locally invasive growth of endometrial seeding lesions and some CA lesions located in the isthmus and in the middle third (m/3) of the endocervical canal were noted, along with the formation of a pedicle and visualized feeding vessels (41%). This finding is described for the first time and has not been analyzed in previous studies. We believe that the neoangiogenesis in the tumor, which is fed by arcuate and large intramural vessels of the myometrium on MRI, indicates aggressive tumor growth, a high probability of lymphovascular invasion (LVI+), an increased probability of lymph node metastasis, and a poor prognosis. However, owing to the small number of reports and the lack of comparison with pathology data (because of the retrospective nature of the data analysis), the characteristics identified are rather observational and require further scientific research.
The ability of CA to seed the endometrium has been reported in several studies and is explained by a “seed and soil” theory, i.e., the detachment of some cancer cells from the primary tumor site, their migration into the uterine cavity, and their implantation in the endometrium [32]. Cell implantation with subsequent formation of feeding vessels and the predominantly uterine location of the growing seeding lesion can be explained by the better blood supply to the myometrium compared to the cervix with dominating fibrous tissue in the stroma.
The use of diffusion-WI and analysis of ADC maps increases the efficiency of this modality compared to standard MRI [33, 34]. Kuang F. et al. showed that ADC is a reliable marker for differentiating CC from normal cervix, with high diagnostic accuracy (ADC for CC was significantly lower than for normal cervix: 0.81 ± 0.13 × 10−3 mm2/sec vs. 1.41 ± 0.10 × 10−3 mm2/sec). Moreover, ADC can be used to determine the grade of differentiation and histologic type of CC. However, there is some overlap between the values. The higher the ACD, the more differentiated the tumor is [34, 35]. In the present study, the mean ADC in the region of interest was 0.833 × 10−3 mm2/sec (range: from 440 × 10−3 mm2/sec; to 1,282 × 10−3 mm2/sec), which is generally consistent with literature data.
There are data on the difference in ADC between cervical squamous cell carcinoma and CA; ADC is significantly lower in squamous cell carcinoma [36].
Lin Y-C. et al. demonstrated that in cases of inconsistent morphology, the tumor ADC can be used to differentiate its histologic type: the mean ADC was significantly lower in EC (0.766 × 10−3 mm2/sec) than in CC (0.969 × 10−3 mm2/sec). In EC, the tumor was reported to have predominantly longitudinal growth in the cervix, whereas in CC, the growth pattern was predominantly oval [37, 38]. Tarachkova EV et al. evaluated semiotic differences between CA and cervical squamous cell carcinoma. In 90 patients with histologically confirmed CC, adenocarcinoma was found to have a more intense and less heterogeneous signal on T2-WI, with fat suppression compared to squamous cell carcinoma [39].
Determining the location of the primary tumor was crucial in the differential diagnosis of uterine adenocarcinoma based on MRI data. Endocervical adenocarcinoma with endometrial involvement and endometrial adenocarcinoma with cervical involvement cannot be distinguished based on signal characteristics in most cases. As this study has shown, one should not rely solely on the location of a larger tumor volume.
In the current study, only 65% (13 of 20) of histologically confirmed endocervical CAs were localized exclusively in the cervix. In 35% of cases (7 of 20), the tumor mimicked EC, with tumor lesions found in the uterine cavity and along the cervical canal. In cases with endocervical adenocarcinoma seeding lesions in the endometrium (3/20, 15%), the size of the tumor implantation in the endometrium exceeded the size of the primary cervical tumor in 2 patients (Fig. 5d). In 2009, a similar pattern in CA growth was reported by Yemelyanova A et al. from the Johns Hopkins University. Scientists evaluated 10 cases of CA with concomitant involvement of the uterine body and cervix and concluded that endometrioid adenocarcinoma with minimal cervical involvement often turned out to be cervical adenocarcinoma [40].
Pathology and immunohistochemistry are objective methods for differential diagnosis of complicated cases of adenocarcinoma of the cervix and uterine body. However, it should be noted that even pathology and immunohistochemistry do not reveal a definitive diagnosis in 100% of cases, as some endometrioid adenocarcinomas are difficult to distinguish from endocervical CA; partial staining of the latter for p16 and estrogen and progesterone receptors is possible [17]. Endometrioid CA should be diagnosed with caution. Generally, endometrioid adenocarcinoma of the cervix and uterine body may have a similar immunohistochemical profile [41]. In the case of simultaneous lesions of the uterine body and cervix, it is crucial to exclude advanced endometrial and ovarian adenocarcinoma and correlate clinical and diagnostic data, as the primary location of the tumor will be critical in the choice of treatment strategy and chemotherapy regimens. According to the IECC, the term “endometrioid carcinoma” refers to tumors with low-grade endometrioid glands and confirmatory endometrioid features (squamous metaplasia or endometriosis) [41].
Therefore, differential diagnosis of complicated cases of adenocarcinoma of the uterine cervix and body should be based on a comprehensive examination of a patient, including history-taking and physical examination, confirmatory morphology, immunohistochemistry, and MRI.
CONCLUSION
Owing to infiltrative tumor growth, frequent location in the upper cervical canal and mucosa of the isthmus, endocervical CA is diagnosed at late stages. This is related to the difficulty in obtaining sufficient cytology material for PAP smear and failure to visualize cervical abnormalities during gynecologic examination, which prevents prompt tumor detection. Late diagnosis of endocervical CA results in the detection of a locally advanced process with frequent direct or metastatic lesions of the endometrium, mimicking primary EC.
This study showed that MRI is an instrumental tool with a high value in the diagnosis of uterine tumors. Four MRI-based macrostructural types of CA growth indicate locally aggressive tumor growth and a high frequency of seeding into the endometrium and allow a radiologist to structure the descriptive image, even in the presence of CA, and subsequently develop a better treatment plan for a patient.
The limitations of MRI in the detection of small cervical tumors are related to the predominantly infiltrative and lateral-spreading tumor growth without increasing the uterine size and changing the endocervical signal characteristics. However, if MRI shows heterogeneous expansion and hyperplasia of the cervical canal, a tumor may be suspected, and the patient should be urgently referred to a gynecologic oncologist. In the absence of clear guidelines for the management of patients with CA in situ, MRI is the preferred method for follow-up. If the primary lesion of uterine adenocarcinoma cannot be determined, a combination of physical (gynecologic) examination, imaging (MRI), and morphologic diagnostic methods should be used.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. I.B. Antonova — concept and design of the study, agreement on the final version of the text; S.P. Aksenova — writing the text of the article, analyzing the results, preparing illustrations, editing the manuscript; N.V. Nudnov — concept and design of the study, approval of the final version of the text; A.V. Krieger — writing the text of the article, analyzing the results, editing the manuscript.
About the authors
Irina B. Antonova
Russian Scientific Center of Roentgenoradiology
Email: Iran24@yandex.ru
ORCID iD: 0000-0003-2668-2110
SPIN-code: 6247-3917
MD, Dr. Sci. (Medicine)
Russian Federation, MoscowSvetlana P. Aksenova
Russian Scientific Center of Roentgenoradiology
Author for correspondence.
Email: fabella@mail.ru
ORCID iD: 0000-0003-2552-5754
SPIN-code: 4858-4627
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowNikolay V. Nudnov
Russian Scientific Center of Roentgenoradiology; Peoples’ Friendship University of Russia; Russian Medical Academy of Continuous Professional Education
Email: nvnudnov@rncrr.ru
ORCID iD: 0000-0001-5994-0468
SPIN-code: 3018-2527
MD, Dr. Sci. (Medicine), Professor
Russian Federation, Moscow; Moscow; MoscowAnna V. Kriger
Russian Scientific Center of Roentgenoradiology
Email: dr.akriger@gmail.com
ORCID iD: 0000-0001-6823-2658
SPIN-code: 2338-6164
Russian Federation, Moscow
References
- Global health estimates: Leading causes of death. Cause-specific mortality, 2000–2019. World Health Organization; c2024. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Kaprin AD, Starinskii VV, Shakhzadova AO, editors. State of oncological care for the Russian population in 2021. Moscow: MNIOI im. P.A. Gertsena — filial FGBU “NMITs radiologii” Minzdrava Rossii; 2022. (In Russ).
- Chan ZF, Zhi KZ. Prevalence and attribution of high-risk HPV in different histological types of cervical cancer. Zhonghua Fu Chan Ke Za Zhi. 2019;54(5):293–300. doi: 10.3760/cma.j.issn.0529-567x.2019.05.002
- Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927–935. doi: 10.1002/ijc.25396
- Nicolás I, Marimon L, Barnadas E, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. 2019;32(8):1189–1196. doi: 10.1038/s41379-019-0249-1
- NCCN guidelines panel. Cervical Cancer. Version 1.2021 PA: National Comprehensive Cancer Network; c2024. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426
- Cervical cancer. Clinical guidelines. ID 537. Approved by the Scientific and Practical Council of the Ministry of Health of the Russian Federation. 2020. Available from: https://cr.minzdrav.gov.ru/recomend/537_1 (In Russ).
- Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol. 2018;42(2):214–226. doi: 10.1097/PAS.0000000000000986
- Grigoruk OG, Moskvina TA, Tsoy DA, et al. Endocervical adenocarcinomas. Cytological, histological, and molecular diagnostics. Tumors of female reproductive system. 2022;18(2):109–118. doi: 10.17650/1994-4098-2022-18-2-109-118
- World Health Organization. Female Genital Tumors. In: WHO Classification of Tumours, 5th Edition, Volume 4. International Agency for Research on Cancer; 2020.
- Islami F, Fedewa SA, Jemal A. Trends in cervical cancer incidence rates byage, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Preventive Medicine. 2019;123:316–323. doi: 10.1016/j.ypmed.2019.04.010
- Hu J, Zheng P, Zhu L. Comparison of clinical pathological characteristics in ovarian preserving patients with stage IB1 cervical adenocarcinoma and squamous cell carcinoma. Journal of Peking University (Health Sciences). 2016;48(5):783–787. doi: 10.3969/j.issn.1671-167X.2016.05.006
- Hu K, Wang W, Liu X, et al. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat Oncol. 2018;13(1):249. doi: 10.1186/s13014-018-1197-5
- Fan Y, Wang M, Mu Y, et al. Ovarian metastasis in women with cervical carcinoma in stages IA to IIB. Medicine (Baltimore). 2020;99(31):e21146. doi: 10.1097/MD.0000000000021146
- Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl. 1:28–44. doi: 10.1002/ijgo.13865
- Stewart CJR, Crum CP, McCluggage WG, et al. Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites. Int J Gynecol Pathol. 2019;38 Suppl. 1(1 Suppl. 1):S75–S92. doi: 10.1097/PGP.0000000000000553
- Song J, Im S, Lee SH, Jang HJ. Deep Learning-Based Classification of Uterine Cervical and Endometrial Cancer Subtypes from Whole-Slide Histopathology Images. Diagnostics (Basel). 2022;12(11):2623. doi: 10.3390/diagnostics12112623
- Woo S, Atun R, Ward ZJ, et al. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: systematic review and meta-analysis. Eur Radiol. 2020;30(10):5560–5577. doi: 10.1007/s00330-020-06909-3
- Merz J, Bossart M, Bamberg F, et al. Revised FIGO Staging for Cervical Cancer — A New Role for MRI. Rofo. 2020;192(10):937–944. doi: 10.1055/a-1198-5729
- Rubtsova NA, Novikova EG, Sinitsyn VE. MRI opportunities in cervical cancer local staging preoperative evaluation. Obstetrics, Gynecology and Reproduction. 2012;6(3):6–13. EDN: PUVJZT
- Balcacer P, Shergill A, Litkouhi B. MRI of cervical cancer with a surgical perspective: staging, prognostic implications and pitfalls. Abdom Radiol (NY). 2019;44(7):2557–2571. doi: 10.1007/s00261-019-01984-7
- Woo S, Suh CH, Kim SY, et al. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. European Radiology. 2018;28(2):530–541. doi: 10.1007/s00330-017-4958-x
- Alt CD, Bharwani N, Danza FM, et al. ESUR Quick Guide to Female Pelvis Imaging. ESUR, 2019. Available from: https://www.researchgate.net/publication/334725882_ESUR_Quick_Guide_to_Female_Pelvis_Imaging
- Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix. Int J Cancer. 2016;139(5):1040–1045 doi: 10.1002/ijc.30152
- Suh DH, Ha HI, Lee YJ, et al. Incidence and treatment outcomes of uterine cervical cancer in Korea 1999–2018 from the national cancer registry. J Gynecol Oncol. 2023;34(2):e39. doi: 10.3802/jgo.2023.34.e39
- Miyamoto T, Kobara H, Shiozawa T. Biology and management of lobular endocervical glandular hyperplasia. J Obstet Gynaecol Res. 2022;48(12):3056–3067. doi: 10.1111/jog.15441
- Kerwin CM, Markese M, Moroney MR, et al. Adenocarcinoma of the uterine cervix, gastric-type (GAS): a review of the literature focused on pathology and multimodality imaging. Abdom Radiol (NY). 2023;48(2):713–723. doi: 10.1007/s00261-022-03724-w
- Marnitz S, Tsunoda AT, Martus P, et al. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB–IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. Int J Gynecol Cancer. 2020;30(12):1855–1861. doi: 10.1136/ijgc-2020-001973
- Akhavan S, Mousavi A, Sheikh Hassani S, et al. Evaluation of Cervical Cancer Staging Based on Magnetic Resonance Imaging in Comparison with Surgical Staging. Int J Cancer Manag. 2023;16(1):e126966. doi: 10.5812/ijcm-126966
- Trukhacheva NG, Frolova IG, Kolomiets LA, et al. Assessment of the extent of cervical cancer spread using magnetic resonance imaging. Siberian journal of oncology. 2015;(2):64–70. EDN: TSLSAD
- Horn LC, Höhn AK, Stark S, et al. Endocervical adenocarcinoma in situ (AIS) with ovarian and pulmonary involvement: report of a case and review of the literature suggesting a “seed and soil hypothesis”. J Cancer Res Clin Oncol. 2019;145(8):2061–2069. doi: 10.1007/s00432-019-02966-4
- Mao L, Zhang X, Chen T, et al. High-resolution reduced field-of-view diffusion-weighted magnetic resonance imaging in the diagnosis of cervical cancer. Quant Imaging Med Surg. 2023;13(6):3464–3476. doi: 10.21037/qims-22-579
- Kuang F, Ren J, Zhong Q, et al. The value of apparent diffusion coefficient in the assessment of cervical cancer. Eur Radiol. 2013;23(4):1050–1058. doi: 10.1007/s00330-012-2681-1
- Wang M, Perucho JAU, Chan Q. Diffusion Kurtosis Imaging in the Assessment of Cervical Carcinoma. Acad Radiol. 2020;27(5):E94–E101. doi: 10.1016/j.acra.2019.06.022
- Monist M, Lewkowicz D, Piętak P, et al. Synchronously occurring endometrioid carcinomas of the uterine corpus and uterine cervix preceded by different precancerous lesions: A case study and a literature review. Pathol Res Pract. 2023;245:154452. doi: 10.1016/j.prp.2023.154452
- Lin YC, Lin G, Chen YR, et al. Role of magnetic resonance imaging and apparent diffusion coefficient at 3T in distinguishing between adenocarcinoma of the uterine cervix and endometrium. Chang Gung Med J. 2011;34(1):93–100.
- Gui B, Lupinelli M, Russo L, et al. MRI in uterine cancers with uncertain origin: Endometrial or cervical? Radiological point of view with review of the literature. European journal of radiology. 2022;153:110357. doi: 10.1016/j.ejrad.2022.110357
- Tarachkova EV, Shorikov MA, Panov VO, et al. Possibilities of multiparametric MRI in the differential diagnosis of histological types of cervical cancer in the preoperative period. Tumors of female reproductive system. 2016;12(2):60–69. doi: 10.17650/1994-4098-2016-12-2-60-69
- Yemelyanova A, Vang R, Seidman JD, Gravitt PE, Ronnett BM. Endocervical adenocarcinomas with prominent endometrial or endomyometrial involvement simulating primary endometrial carcinomas: utility of HPV DNA detection and immunohistochemical expression of p16 and hormone receptors to confirm the cervical origin of the corpus tumor. Am J Surg Pathol. 2009;33(6):914–924. doi: 10.1097/PAS.0b013e3181971fdd
- Jain P, Aggarwal A, Ghasi RG, et al. Role of MRI in diagnosing the primary site of origin in indeterminate cases of uterocervical carcinomas: a systematic review and meta-analysis. Br J Radiol. 2022;95(1129):20210428. doi: 10.1259/bjr.20210428
Supplementary files