Unilateral pulmonary vein atresia: Difficulties of radiological diagnosis
- Authors: Zharikova V.V.1, Nechaev V.A.1, Kulikova E.A.1, Yudin A.L.2
-
Affiliations:
- Moscow City Oncological Hospital No. 1
- The Russian National Research Medical University named after N.I. Pirogov
- Issue: Vol 5, No 2 (2024)
- Pages: 361-369
- Section: Case reports
- Submitted: 25.10.2023
- Accepted: 06.02.2024
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/619643
- DOI: https://doi.org/10.17816/DD619643
- ID: 619643
Cite item
Abstract
Pulmonary vein atresia is a rare congenital abnormality that could manifest in isolation or in association with other congenital abnormalities in the cardiovascular system such as pulmonary vein hypoplasia. Pulmonary vein atresia leads to changes in cardiovascular functioning. This abnormality is often diagnosed in children with recurrent pneumonia and hemoptysis. In adulthood, pulmonary vein atresia is much less common, with clinical symptoms such as dyspnea during physical exercises and hemoptysis. However, some patients are asymptomatic. Owing to the nonspecific imaging findings, lung parenchymal changes are often misdiagnosed as another lung disease, including inflammatory genesis disease. In this article, a case of a young man with asymptomatic unilateral pulmonary vein atresia combined with pulmonary artery hypoplasia and interstitial lung changes in a lung with hypoplasia was presented. These pathologies were first identified in a 21-year-old patient by contrast-enhanced computed tomography.
Full Text
INTRODUCTION
Pulmonary vein atresia is a congenital malformation that commonly manifests in infants and young children with recurrent hemoptysis and pneumonia [1]. It is extremely rare in adults, with less than 40 cases of asymptomatic unilateral pulmonary vein atresia newly diagnosed in adults reported in foreign studies [2]. No such cases have been described in Russian studies. This study presents a case of a 21-year-old male with right pulmonary vein atresia associated with hypoplasia of the right pulmonary artery and interstitial lesions in the right lung first identified with computed tomography (CT).
DESCRIPTION OF THE CASE
Patient V, 21 years old, was referred to the City Clinical Oncology Hospital No. 1 of the Moscow City Health Department for a routine examination because of a history of left kidney cancer (pT3N0M0) and status post left nephrectomy for nephroblastoma in 2003.
Medical History
In 2020, a tumor was found in the solitary right kidney. However, immunohistochemistry performed at another healthcare facility showed no evidence of cancer. The patient was followed up by an endocrinologist for Denys–Drash syndrome (karyotype 46, XY; gonadectomy for gonadal dysgenesis), hypergonadotropic hypogonadism, gynecomastia, and short stature. The patient reported a history of heart surgery in childhood. No additional data on examinations and diagnostic and therapeutic interventions performed before 2020 were available.
Physical Examination, Laboratory Tests, and Investigations
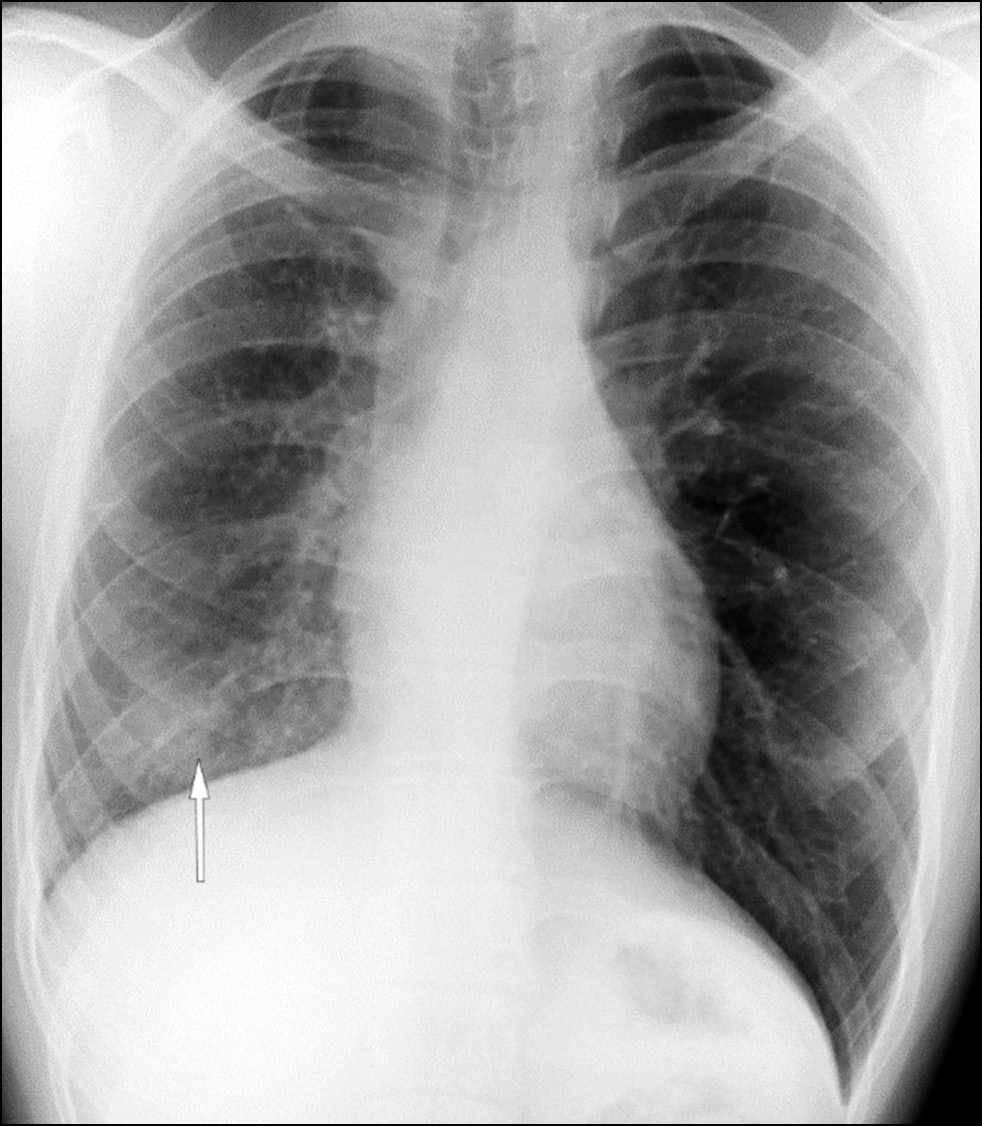
In 2020, a routine chest X-ray showed decreased right lung volume and a shaded, ill-defined lesion in the lower part of the right lung (Fig. 1). During examination, the patient presented no complaints.
Fig. 1. Lung X-ray. Decreased volume of the right lung; a shaded ill-defined lesion in the lower part of the right lung field (white arrow).
Chest CT without contrast was performed to further diagnose the abnormalities. The scan showed interstitial lesions in the right lung, presenting as thickened intra- and interlobular septa, mainly in the middle and lower parts of the right lung, and thickened bronchial walls. Moreover, in the mediastinum, an irregular soft tissue conglomerate was detected in the right tracheobronchial and subcarinal lymph nodes, with a homogeneous structure and density of +40 HU. The findings indicated intrathoracic lymphadenopathy and interstitial disease of the right lung with evidence of bullous emphysema (Fig. 2).
Fig. 2. Computed tomography of the lungs; (a, b) axial plane; (c) sagittal plane; (d) coronal plane. The volume of the right lung is decreased. White arrows: thickened interlobular interstitium. Yellow arrows: lung cyst. Green arrow: homogeneous soft tissue conglomerate with a density of +40 HU in the mediastinum. Computed tomography in 2023 showed no changes over time.
Owing to the lung CT findings, the patient was referred to a pulmonologist for further consultation, and a pulmonary function test was conducted. Decreased respiratory function of the lungs and restrictive and obstructive defects (forced expiratory volume in 1 second was 53%) were detected. In the absence of clinical manifestations, watchful waiting and consultation with an oncologist regarding the right kidney mass and mediastinal lymphadenopathy were recommended.
In 2023, after consultation with an oncologist, the patient was referred for laboratory tests (results within reference limits). As part of watchful waiting, chest, abdominal, and pelvic CT was performed with intravenous contrast. The scan showed persisting decreased volume of the right lung with significant interstitial abnormalities, ground-glass opacity, and cysts in the affected lung. No changes from the previous scan dated 2020 were noted.
Arterial and venous phase scanning along the margins of the right main bronchus revealed multiple dilated, tortuous arterial and venous vessels (bronchial and intercostal) in the intrathoracic lymph nodes; without contrast enhancement, they were previously interpreted as manifestations of intrathoracic lymphadenopathy (Fig. 3). The decreased diameter of the right pulmonary artery to 7 mm (vs. 14 mm on the contralateral side) and absence of contrast enhancement in the right pulmonary veins were clearly visualized in the three-dimensional reconstruction of the heart (Fig. 4).
Fig. 3. Computed tomography of the lungs; (a, b) axial plane; c: coronal plane. White arrows: multiple vascular collaterals along the bronchial contour. Black arrow: hypoplasia of the right pulmonary artery.
Fig. 4. Absence of the right pulmonary veins (white arrows); (a) computed tomography of the lung in the coronal plane; (b) computed tomography of the lung in the axial plane; (c) three-dimensional reconstruction of the heart.
DISCUSSION
Pulmonary vein atresia is a rare congenital malformation, with an incidence of 1.7 cases per 100,000 children aged >2 years [3]. It is believed to occur during intrauterine development when the common pulmonary vein fails to incorporate into the left atrium [4–6]. In most cases, this abnormality is diagnosed in infants and young children with recurrent pneumonia and/or hemoptysis [1, 7, 11]. This malformation can occur on either side, without left or right predominance, and is often associated with a cardiovascular disease, as in our patient with a history of heart surgery in childhood. Additionally, in our case, indirect signs of tricuspid regurgitation were detected, such as blood reflux into the hepatic portion of the inferior vena cava and hepatic veins.
In adults, the main complaints include shortness of breath on exertion and hemoptysis, often associated with pulmonary hypertension [5]. However, in rare cases, the disease may be asymptomatic [3, 8].
In 50% of cases, pulmonary vein atresia is isolated; in the remaining cases, it is associated with other malformations, such as pulmonary artery hypoplasia, resulting in hypoperfusion and decreased size of the affected lung [9, 12]. Pulmonary vascular malformations cause vascular collaterals to form, characterized by dilated intercostal and bronchial arteries and veins. They fuse with newly formed vessels, resulting in thickened interlobular septa, perifissural lesions, and ground-glass opacity as a manifestation of lymphostasis and venous congestion [2, 9]. When blood flow in individual capillaries, small arteries, and veins stops and the vascular network is dilated and blood overflows into these vessels due to impaired normal outflow, the affected lung parenchyma is inadequately supplied with blood. Consequently, the lung tissue is compacted, and the alveolar capillary membrane and interalveolar septa thicken due to increased connective tissue proliferation. This leads to impaired pulmonary gas exchange and hypoxemia [10].
In the present case, cysts were found in the affected lung. This was not an incidental finding, but a consequence of the destruction or underdevelopment of capillary networks at the alveolar level due to insufficient blood supply [2, 7].
Among the primary noninvasive diagnostic methods for congenital cardiovascular abnormalities, echocardiography is crucial for determining anatomical variability, size of the main pulmonary vessels, and blood supply parameters. In the present case, this examination was scheduled; however, the patient did not show up.
A standard chest X-ray may show the decreased volume of the affected lung and increased pulmonary vascularity due to the reticular component. Follow-up noninvasive diagnostics may include CT angiography and heart magnetic resonance imaging (MRI) [9]. In our case, heart MRI was not performed because CT visualized both venous and arterial abnormalities, allowing the diagnosis of venous insufficiency without using other modalities. Moreover, contrast-enhanced CT showed the absence of pulmonary veins, pulmonary artery hypoplasia, decreased size of the right lung, and interlobular septa thickening due to venous congestion.
Unilateral pulmonary venous atresia is a rare congenital malformation often confused with secondary malignancy, pneumonia, and pulmonary tuberculosis [3, 9]. In case of clinical symptoms, pneumonectomy or lung transplantation is recommended [9].
Conclusion
Unilateral pulmonary vein atresia is a rare congenital malformation that is often associated with other cardiovascular abnormalities. A rare case of combined asymptomatic congenital malformations of the pulmonary vessels in a young man is presented. In some cases, mediastinal and pulmonary lesions found in such patients may be misinterpreted as a manifestation of pneumonia, pulmonary tuberculosis, or cancer. However, a combination of radiologic signs on contrast-enhanced chest CT may be beneficial in the early diagnosis of this malformation.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. V.V. Zharikova — clinical evaluation of CT results, data processing, writing text, text editing, preparing illustrations for the article; V.A. Nechaev — clinical evaluation of CT results, text editing, preparing illustrations for the article, advisory support, approval of the final version of the text; E.A. Kulikova — clinical evaluation of CT results, advisory support; A.L. Yudin — text editing, advisory support, advisory support, approval of the final version of the text.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript Digital Diagnostics Journal.
About the authors
Veronika V. Zharikova
Moscow City Oncological Hospital No. 1
Author for correspondence.
Email: ZharikovaVV@zdrav.mos.ru
ORCID iD: 0009-0007-1659-8325
Russian Federation, Moscow
Valentin A. Nechaev
Moscow City Oncological Hospital No. 1
Email: NechaevVA1@zdrav.mos.ru
ORCID iD: 0000-0002-6716-5593
SPIN-code: 2527-0130
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowEvgenia A. Kulikova
Moscow City Oncological Hospital No. 1
Email: kulikovaEA14@zdrav.mos.ru
ORCID iD: 0000-0002-0319-4934
SPIN-code: 2884-4803
Russian Federation, Moscow
Andrey L. Yudin
The Russian National Research Medical University named after N.I. Pirogov
Email: prof_yudin@mail.ru
ORCID iD: 0000-0002-0310-0889
SPIN-code: 6184-8284
MD, Dr. Sci. (Medicine), Professor
Russian Federation, MoscowReferences
- Patil PP. Right pulmonary venous atresia: a rare cause for recurrent unilateral pneumonia. J. Clin. Diagn. Res. 2017;11(9):SD01–SD02. doi: 10.7860/JCDR/2017/25670.10596
- Kim Y, Yoo IR, Ahn MI, Han DH. Asymptomatic adults with isolated, unilateral right pulmonary vein atresia: multidetector CT findings. Br. J. Radiol. 2011;84(1002):109–113. doi: 10.1259/bjr/51344661
- Cohn H-ER, Hicks M, Lacson A, Hicks A. Left hypoplastic lung and hemoptysis — rare familial unilateral pulmonary vein atresia. Clin. Case Rep. 2020;8(9):1698–1703. doi: 10.1002/ccr3.2982
- Reller MD, McDonald RW, Thornburg K, et al. Cardiac embryology: basic review and clinical correlations. J. Am. Soc. Echocardiogr. 1991;4(5):519–532. doi: 10.1016/s0894-7317(14)80388-x
- Heyneman LE, Nolan RL, Harrison JK, McAdams HP. Congenital unilateral pulmonary vein atresia: radiologic findings in three adult patients. Am. J. Roentgenol. 2001;177(3):681–685. doi: 10.2214/ajr.177.3.1770681
- Dixit R, Kumar J, Chowdhury K, et al. Case report: isolated pulmonary vein atresia diagnosted on 128-slice multidetector CT. Indian J. Radiol. Imaging. 2011;21(4):253–256. doi: 10.4103/0971-3026.90681
- Lee SC, Yi JG, Park JH. Cystic lung changes in a thin section CT in an asymptomatic young adult with unilateral pulmonary vein atresia: a case report. J. Korean Soc. Radiol. 2012;67(1):45–48. doi: 10.3348/jksr.2012.67.1.45
- Park S, Cha YK, Kim JS, et al. Isolated Unilateral Pulmonary Artery Hypoplasia with Accompanying Pulmonary Parenchymal Findings on CT: A Case Report. J. Korean Soc. Radiol. 2017;76(5):369–373. doi: 10.3348/jksr.2017.76.5.369
- Cong C-V, Ly T-T, Duc NM. Unilateral pulmonary vein atresia: Literature overview and case report. Radiol. Case Rep. 2022;17(4):1313–1317. doi: 10.1016/j.radcr.2022.01.057
- Pavlenko SM. Pathological physiology. Moscow: Medgiz; 1940. (In Russ).
- Basavarai B, Arun S, Amarinder SM, et al. Unilateral pulmonary vein atresia: diagnostic dilemma unfolded on imaging. BMJ Case Rep. 2018. doi: 10.1136/bcr-2017-224154
- Narayanan R, Shankar B, Paruthikunnan S. Isolated unilateral pulmonary vein atresia. Lung India. 2016;33(5):571–572. doi: 10.4103/0970-2113.188990
Supplementary files