Mitral valve calcinosis as an important finding during heart examination
- Authors: Filatova D.A.1, Mershina E.A.1, Plotnikova M.L.1, Lisitskaya M.V.1, Sinitsyn V.E.1
-
Affiliations:
- Lomonosov Moscow State University
- Issue: Vol 5, No 2 (2024)
- Pages: 219-230
- Section: Original Study Articles
- Submitted: 17.12.2023
- Accepted: 24.01.2024
- Published: 20.09.2024
- URL: https://jdigitaldiagnostics.com/DD/article/view/624754
- DOI: https://doi.org/10.17816/DD624754
- ID: 624754
Cite item
Abstract
BACKGROUND: Mitral valve calcinosis is a chronic degenerative process in the fibrous structures of the mitral valve. Advanced stages increase the risk of endocarditis and cardiac rhythm disturbances and contribute to cardiovascular mortality. The cause of mitral valve calcinosis is still controversial; however, the contribution of atherosclerosis to its development is currently undisputed. The prevalence of mitral valve calcinosis varies in different age groups and on average is higher in people with cardiovascular disease.
AIM: To assess the prevalence of mitral valve calcinosis in patients undergoing computed tomography angiography and identify the relationship between aortic and mitral valve calcinosis and coronary calcium index and signs of remodeling.
MATERIALS AND METHODS: A retrospective study of 336 patients who underwent computed tomography coronary angiography with intravenous contrast enhancement at the Lomonosov Moscow State University Clinic between November 13, 2020, and May 14, 2022, was conducted.
RESULTS: The prevalence of aortic (16.4%) and mitral (11%) valve calcinosis was high in people undergoing cardiovascular examination, and a relationship was noted between valve calcinosis and coronary calcium index.
CONCLUSION: The detection of mitral valve calcinosis in patients during routine examination is important in predicting further treatment and outcomes because valve calcinosis is an indirect indicator of coronary heart disease risk. Although valve calcinosis is usually an incidental examination finding, it may indicate a high cardiovascular risk and should prompt further evaluation, if clinically necessary.
Full Text
Background
Mitral valve calcinosis (MVC) is a chronic degenerative condition of the fibrous ring of the mitral valve (MV). It is characterized by calcium deposition, primarily in the posterior leaflet. Caseous calcinosis is a distinct type of MV damage, with a biochemical transformation of calcium and the formation of caseous masses (thus the name). MVC (ordinary or caseous) is frequently mistakenly regarded as a neoplasm based on imaging findings; thus, the appropriate use of different methods is critical for the differential diagnosis.
The overall incidence of MVC is approximately at 13% [1]; however, it can range from 4.6% to 15.8%, depending on the population [2]. The incidence of MVC is 35% in patients with symptoms of cardiovascular diseases [3] and 36% in patients with advanced renal insufficiency [1]. MVC is typically asymptomatic and is discovered by accident during examinations, contributing to this substantial variability. The Framingham Heart Study revealed that MVC is extremely rarely detected in patients aged <40 years [4]. This has a major effect on cardiovascular morbidity and mortality and outcomes of cardiovascular surgery. Unlike rheumatic diseases, calcinosis usually does not significantly impair MV function; however, severe MVC can cause mitral regurgitation.
STUDY AIM
This study aimed to assess the incidence and characteristics of MVC and aortic valve calcinosis (AVC) in patients who were tested for coronary artery atherosclerosis using computed tomography (CT) coronary angiography. Moreover, the study assessed the potential relationship between these processes and the effect of calcinosis on MV function and cardiac chamber morphology.
Methods
Study Design
This retrospective, observational, single-center cohort study enrolled patients who underwent CT coronary angiography in the X-ray diagnosis department of the Medical Research and Educational Center of the Lomonosov Moscow State University.
Eligibility Criteria
Inclusion criteria: patients referred for contrast-enhanced CT coronary angiography to rule out coronary artery atherosclerosis.
Noninclusion criteria:
- Condition after coronary artery stenting or bypass grafting (calcium score assessment was not feasible)
- Condition after valve replacement
- Congenital heart disorders and heart neoplasms
- History of severe allergic reaction to an iodine-containing contrast enhancement agent
- Severe condition preventing a diagnostically valuable examination
Exclusion criteria: refusal to participate in the study.
Study Duration
The study was performed between November 13, 2020, and May 14, 2022.
Therapeutic Intervention
CT coronary angiography with electrocardiogram (ECG) gating was performed, which included two successive scans: a native scan to determine the cardiac calcium score (CCS) and an arterial enhancement scan (using an iodine-containing contrast enhancement agent at a dose of 300 mg iodine per 1 mL, or 1 mL per 1 kg body weight). Retrospective ECG gating was employed in all cases, which enabled additional visualization of cardiac chambers during various phases of the cardiac cycle. Coronary artery reconstruction in the multiplanar reformation (MPR) mode was used for the analysis of images.
Primary Outcome
Detection of MVC and AVC and calcified plaques in coronary artery walls.
Secondary Outcomes
Detection of cardiac remodeling signs (left atrial enlargement).
Subgroup Analysis
The patients were divided into subgroups depending on the presence of valvular calcinosis:
- MVC group
- AVC group
- MVC and AVC group
- Group without valvular calcinosis
Moreover, the patients were divided into subgroups depending on the CCS:
- 0 (no coronary artery lesion)
- 1–10 (minimal lesion)
- 11–100 (insignificant lesion)
- 101–400 (moderate lesion)
- >400 (severe lesion)
Ethics Review
The study was approved by the Ethics Committee of the Medical Research and Educational Center of the Lomonosov Moscow State University on September 24, 2020.
Statistical Analysis
Statistical analysis was performed using Microsoft Office Excel 2010 (Microsoft Corporation, USA). Data were analyzed using the Kruskal–Wallis, Mann–Whitney U, and chi-squared tests. The significance level was P < 0.05.
Results
Study Subjects
The study included 336 patients, of which 195 were men (58%). The mean age was 61.1 ± 11.8 years.
Primary Findings
The incidence rates of MVC and AVC in the study groups were 11% (n = 37) and 16.4% (n = 55), respectively. Calcification of both valves was observed in 3.9% of cases (n = 13). Caseous MVC was reported in 7 (2.1%) patients.
Table 1 provides information on the study groups and the detected incidence of valvular calcinosis.
Table 1. Incidence of valvular calcification in the study groups
MVC | AVC | MVC, AVC | No calcification | |
Number of patients | 37 | 55 | 13 | 251 |
Mean age, years | 66.1±8.9 | 66.7±7.9 | 67.0±9.7 | 60.1±11.6 |
Men/women | 16/21 | 32/23 | 5/8 | 149/102 |
CCS, units | 582.3±875.3 | 435.3±591.4 | 314.2±215.2 | 176.5±402.9 |
Note. AVC, aortic valve calcification; MVC, mitral valve calcification; CCS, cardiac calcium score.
When dividing patients into groups depending on the presence of valvular calcinosis, significant differences in the CCS were revealed. The analysis was performed using the Kruskal–Wallis test (P < 0.001). The MVC group had the highest CCS, whereas the group without calcinosis had the lowest value.
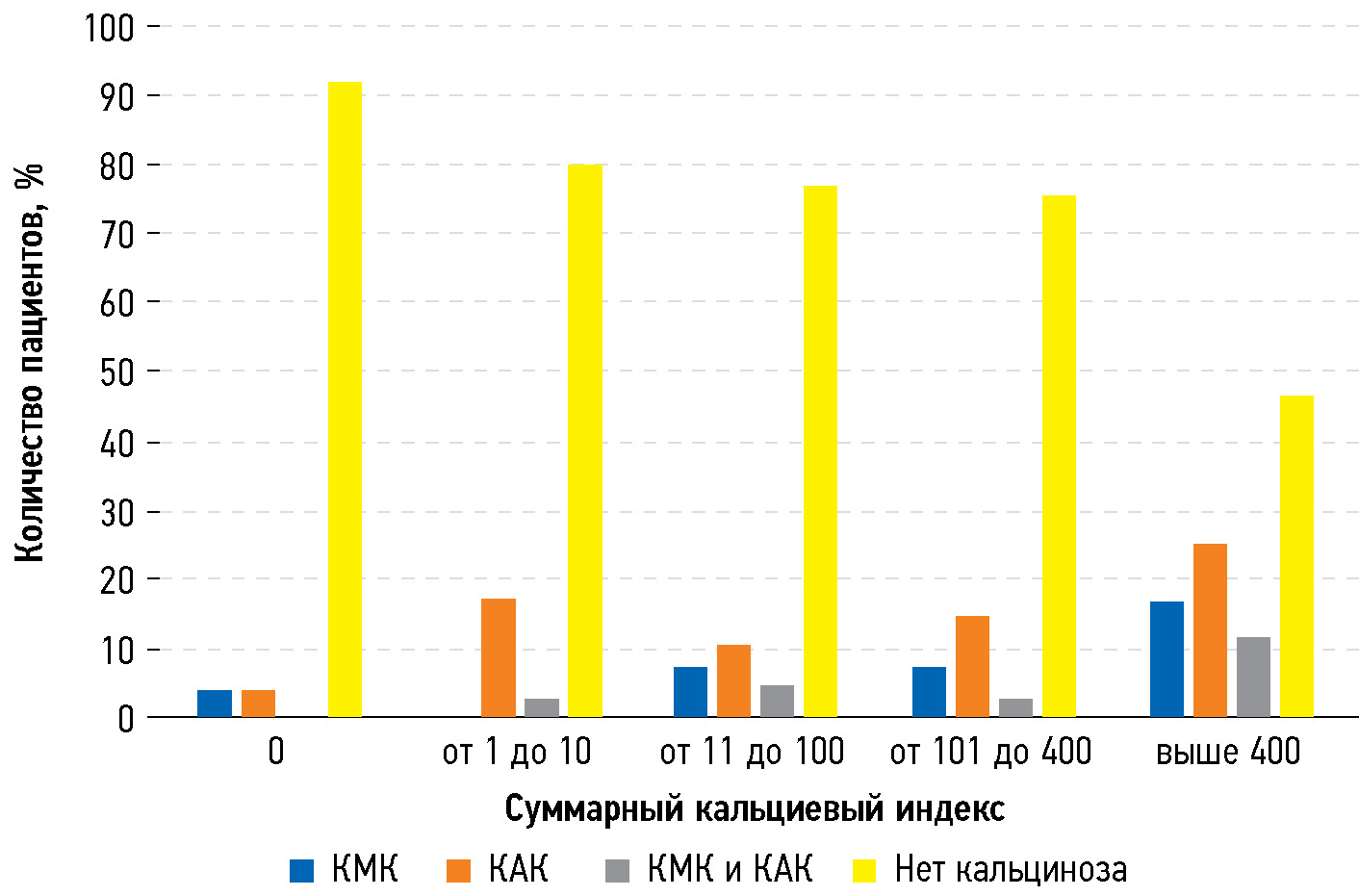
When analyzing arbitrary-sized contingency tables using the chi-squared test, a positive correlation was found between the CCS and the incidence of MVC and AVC (P < 0.01) (Fig. 1). However, the association was less significant for AVC compared with that for MVC.
Fig. 1. Number of patients with valvular calcification in groups with different cardiac calcium score: AVC, aortic valve calcification; MVC, mitral valve calcification.
The severity of valvular calcinosis was assessed by calculating the CCS and the estimated calcium volume. When comparing the coronary artery CCS and severity of valvular calcinosis using the Mann–Whitney U test, no significant association was found. This pattern was observed in both the MVC and AVC groups.
Additional Findings
A potential association between left atrial enlargement (an indirect factor of mitral valve disease) and MVC was assessed. The analysis using the chi-squared test revealed a significant association between the MVC factor and left atrial enlargement as an outcome (P = 0.002).
Adverse Events
No adverse events or side effects of the contrast agent were reported during the study.
Discussion
Summary Of Primary Findings
This retrospective study demonstrated a high incidence of MVC and AVC in patients undergoing a cardiovascular examination using CT coronary angiography and an association between valvular calcinosis and the CCS, with a more significant correlation for MVC. However, the severity of calcinosis was not associated with the coronary artery CCS. Moreover, a relationship was found between MVC and left atrial enlargement.
Discussion of Primary Findings
According to the literature, a correlation was found among MVC, AVC, and coronary artery atherosclerosis [5]. Moreover, a correlation was noted between coronary artery atherosclerosis and systemic atherosclerosis. Our findings are of relevance given that AVC exhibited a less significant correlation with the CCS than MVC. This emphasizes that the likelihood of valvular calcinosis is determined not only by systemic atherosclerosis and associated risk factors but also by other conditions. Timely diagnosis of MVC is clinically significant because MVC can be considered a predictor of atherosclerosis and MV disease in the future.
The pathophysiology of MVC is not fully understood. Nestico et al. defined MVC as a chronic, age-related degenerative condition that affects the fibrous support structure of the MV [6]. During the first decade of life, the mitral annulus is made up of parallel thin collagen fibers and some elastic fibers. The thickness and density of collagen fibers increase with age, as does the number of elastic fibers. Lipid spots form between collagen fibers, accompanied by calcinosis areas. Over time, collagen fiber misalignment increases, and lipid and calcium deposits grow. Areas with decreased shear stress and increased blood flow turbulence are susceptible to atherosclerosis [7]. Calcification and lipid deposition in the mitral annulus are commonly reported during autopsy in patients without macroscopic signs of MVC [8].
Studies have demonstrated significant similarities between atherosclerotic cardiovascular disease and chronic degenerative changes in the mitral and aortic valves [9]; all these events are typically caused by endothelial damage or dysfunction [10]. Moreover, as calcinosis forms, the mitral valve changes typical of atherosclerosis, such as inflammation [11], lipid buildup [12], and matrix metalloproteinase activation [13]. The renin–angiotensin–aldosterone system also plays a role in the development of valvular calcinosis [14]. Over time, focal calcium and lipoprotein deposits at microdamage areas transform into dense, rigid structures that form MVC. Moreover, numerous studies have demonstrated a significant correlation between the severity of systemic atherosclerosis and associated risk factors with MVC [15–17]. Thus, several researchers propose that MVC and atherosclerotic cardiovascular disease are two variants of the same condition.
However, an essential feature of MVC pathogenesis is that MVC more frequently affects women, whereas classic atherosclerosis is more common in men [18]. Women also have larger deposits in the mitral annulus. The formation of extrauterine calcium deposits in older women was assumed to be associated with severe bone loss due to osteoporosis in postmenopause [19]. Moreover, bisphosphonate therapy is associated with a lower incidence of cardiovascular calcinosis in women aged >65 years [20].
In addition to atherosclerosis, conditions associated with a long-term MV overload, such as hypertension, aortic stenosis, and hypertrophic cardiomyopathy, are also significant contributors to MVC development. In these conditions, the peak systolic pressure and MV closing pressure increase, resulting in an increased load on the mitral annulus and causing its degeneration [21].
Chronic renal failure is another significant risk factor for MVC. Reduced glomerular filtration rate, end-stage renal disease, and hemodialysis are linked to the incidence of MVC [22]. This association can be attributable to various reasons, including a high incidence of cardiovascular risk factors and atherosclerosis in these patients, more severe comorbidities, and impaired calcium–phosphorus metabolism [23, 24]. Jesri et al. demonstrated that nearly 60% of patients with MVC have a reduced glomerular filtration rate of ≤60 mL/ min × 1.73 m2 [25]. Notably, no such association was found between chronic kidney disease and MVC [26]. Moreover, patients with specific congenital disorders, such as Marfan syndrome, which is characterized by systemic connective tissue and cardiovascular system damage, are more susceptible to MVC [27].
The Framingham Heart Study revealed a correlation between MVC and the incidence of adverse cardiovascular events and cardiovascular mortality [28]; the risk of the latter depends on the severity of MVC [17]. The main reason is that MVC is considered a marker of systemic atherosclerosis and coronary artery atherosclerosis [29]. Inflammatory, immune, and metabolic processes can also participate in this relationship. MVC in patients aged <65 years with chest pain is an important, independent predictor of significant stenosis of at least one coronary artery. The absence of MVC in women aged <65 years is an independent predictor of the absence of coronary heart disease [3]. Thus, MVC in patients aged <65 years must be considered by practitioners as a significant marker of coronary heart disease. It is relevant in patients without clinical signs of this disease, and these patients require risk factor and lifestyle modifications to reduce the risk of adverse coronary events.
The literature data on the relationship between MVC and stroke are less consistent. Some studies (e.g., Kizer et al. [30]) confirm this correlation, whereas others (Rodriguez et al. [31]) found that it significantly decreased after making adjustments for classic risk factors for stroke. MVC is associated with coronary artery atherosclerosis and ventricular fibrillation, which can explain the reported correlation between MVC and stroke [32, 33].
Evidence shows that calcinosis can affect the MV function because the mobility of the posterior leaflet decreases when the base is densely infiltrated. This, in turn, increases the risk of elongation and rupture of the chordae tendineae, resulting in secondary mitral regurgitation [34]. The incidence of endocarditis in MVC is unknown. Although it is rare, it is a potentially fatal condition characterized by vegetation on the MV leaflets or mitral annulus [35].
The association between MVC and cardiac rhythm disorders is well-known. The most common disorders are atrioventricular block, bundle branch block, and intraventricular conduction block [36]. According to the literature, the incidence of cardiac rhythm disorders was significantly higher in patients with MVC than in the control group (70% vs. 34%) [37]. This could be because calcinosis directly extends to the atrioventricular node and the bundle of His. A significant correlation between MVC and atrial fibrillation was also associated with left atrial enlargement [38].
Given that MVC is mostly asymptomatic and can have potentially life-threatening consequences, its timely diagnosis is critical. In some cases, distinguishing between MVC, thrombosis, and neoplasms is difficult. Moreover, some authors report that calcinosis may appear similar to a myocardial abscess [39].
Echocardiography (EchoCG) is used for the primary diagnosis of MVC, which is visualized as a static hyperechoic structure with sharp edges, usually in the subvalvular space, beneath the MV posterior leaflet. Moreover, MVC is sometimes visualized as a heterogeneous structure with hypoechoic areas. Large calcinosiss may form an acoustic shadow, preventing clear visualization. Observation of typical MVC characteristics does not require further examination; however, the sensitivity of EchoCG is often insufficient to distinguish between calcium and other dense structures (such as collagen). Advanced abscesses can resemble MVC caused by consolidation and calcinosis areas. Magnetic resonance imaging (MRI) and CT can be used for follow-up examinations in cases in which visualization using EchoCG is difficult when there is a nonspecific increase in inflammatory markers (which does not allow ruling out an abscess or neoplasm), and in some other doubtful situations.
MVC typically has a decreased signal on standard cine MRI sequences, which sometimes makes it difficult to distinguish between calcium, adjacent cardiac muscles, and neoplasms in this location. T2-weighted images make it easier to differentiate MVC from other surrounding structures. Contrast enhancement does not result in significant perfusion during the early phase or delayed enhancement during the late phase (Fig. 2c). A thin rim of contrast is sometimes observed along the margin during the late phase. MVC can be distinguished from benign or malignant neoplasms based on the absence of vascularization and necrosis in the central part. MVC is displayed as a hypointense central part on T1- and T2-weighted images, whereas myxoma and lipoma are characterized by a hypointense signal due to mucin or fat in the stroma [40]. MR signs of abscesses depend on the stage; however, they are typically visualized as structures with a hypointense signal in the center and a hypointense signal in the periphery. However, the sensitivity and specificity of MRI are not always sufficient for an accurate differential diagnosis among MVC, thrombosis, and tumors. In these cases, CT is employed for follow-up examination.
Fig. 2. Female patient, aged 65 years. Mitral valve calcification according to various methods: (a, b) computed tomography (axial and sagittal planes, without contrast enhancement); (c) magnetic resonance imaging (four-chamber long-axis view, delayed contrast enhancement).
On CT, MVC is visualized as a hyperdense structure without signs of contrast uptake, with an avascular, “soft” center. A fibrous capsule with heterogeneous, dense calcium areas is sometimes observed in the periphery [41] (Fig. 2a, b). CT allows determining the location and extent of MVC and its effect on valve function. Calculating the CCS enables a quantitative assessment of MVC.
Thus, a multimodal approach is recommended to make an accurate diagnosis of MVC. EchoCG is the first-line method, and ambiguous results necessitate additional examination using both MRI and CT. Notably, the use of various imaging methods not only allows for a correct diagnosis but also provides valuable information about the other conditions (coronary artery atherosclerosis, valve stenosis, reduced ventricular contractility, reduced ventricular contractility, hypokinesia or dyskinesia areas, etc.) of the patient, which may affect the treatment strategy.
Study Limitations
The study limitations include the analysis of calcified coronary artery plaques solely disregarding soft plaques. The correlation between the severity of coronary artery stenosis and soft plaques will be assessed in the next phase of the study. In addition, clinical data, as well as EchoCG findings, were not analyzed.
Conclusion
MVC is commonly observed and is typically asymptomatic. The etiology of this condition is not fully understood; however, it shows a clear association with systemic atherosclerosis. MVC is associated with atherosclerosis and MV disease; thus, early disease diagnosis is clinically significant for the prevention of potentially dangerous conditions.
This study revealed a high incidence of valvular calcinosis in the population (the incidence rates of MVC, AVC, and calcinosis of both valves were 11%, 16.4%, and 3.9%, respectively). Moreover, a correlation was found between valvular calcinosis and coronary artery atherosclerosis, with a more significant association for the MV. Moreover, a relationship was found between MVC and left atrial enlargement.
Distinguishing between MVC, heart neoplasms, thrombosis, and some other conditions is difficult. EchoCG is used for the primary diagnosis of MVC; however, this condition can be mistaken for a neoplasm or thrombosis. Thus, when the findings are insufficient, follow-up examinations using cardiac MRI and CT are recommended. The use of a comprehensive approach and ensuring appropriate follow-up in patients with MVC and concomitant conditions, including calcinosis of other heart valves, are encouraged.
ADDITIONAL information
Funding source. The work was performed within the framework of the State assignment of Lomonosov Moscow State University (theme 0708 “Application of new functional and perfusion CT and MRI techniques to improve diagnostics”).
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
D.A. Filatova — forming the research group, conducting data statistical analysis, writing the text of the article; E.A. Mershina — developing the research concept, editing the text of the article; M.L. Plotnikova — editing the text of the article; M.V. Lisitskaya — development of the research concept, editing the text of the article; V.E. Sinitsyn — development of the research concept, approval of the final version of the text.
About the authors
Daria A. Filatova
Lomonosov Moscow State University
Author for correspondence.
Email: dariafilatova.msu@mail.ru
ORCID iD: 0000-0002-0894-1994
SPIN-code: 2665-5973
MD
Russian Federation, MoscowElena A. Mershina
Lomonosov Moscow State University
Email: elena_mershina@mail.ru
ORCID iD: 0000-0002-1266-4926
SPIN-code: 6897-9641
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowMaria L. Plotnikova
Lomonosov Moscow State University
Email: maria_plotnikova@inbox.ru
ORCID iD: 0000-0001-7533-9867
SPIN-code: 1857-0770
MD
Russian Federation, MoscowMariya V. Lisitskaya
Lomonosov Moscow State University
Email: lissenok@inbox.ru
ORCID iD: 0000-0002-8402-7643
SPIN-code: 2301-8480
MD, Cand. Sci. (Medicine)
Russian Federation, MoscowValentin E. Sinitsyn
Lomonosov Moscow State University
Email: vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-code: 8449-6590
MD, Dr. Sci. (Medicine), Professor
Russian Federation, MoscowReferences
- Maher ER, Young G, Smyth-Walsh B, Pugh S, Curtis JR. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;330(8564):875–877. doi: 10.1016/s0140-6736(87)91370-5
- Fox E, Harkins D, Taylor H, et al. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am. Heart J. 2004;148(6):979–984. doi: 10.1016/j.ahj.2004.05.048
- Atar S, Jeon DS, Luo H, Siegel RJ. Mitral annular calcification: a marker of severe coronary artery disease in patients under 65 years old. Heart. 2003;89(2):161–164. doi: 10.1136/heart.89.2.161
- Savage DD, Garrison RJ, Castelli WP, et al. Prevalence of submitral (anular) calcium and its correlates in a general population-based sample (the Framingham Study). Am. J. Cardiol. 1983;51(8):1375–1378. doi: 10.1016/0002-9149(83)90315-6
- Barasch E, Gottdiener JS, Larsen EKM, et al. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am. Heart J. 2006;151(1):39–47. doi: 10.1016/j.ahj.2005.03.052
- Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am. Heart J. 1984;107(5 Pt 1):989–996. doi: 10.1016/0002-8703(84)90840-8
- Stary HC, Blankenhorn DH, Chandler AB, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. J. Vasc. Biol. 1992;12(1):120–134. doi: 10.1161/01.atv.12.1.120
- Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and Aortic Annular Calcification Are Highly Associated With Systemic Calcified Atherosclerosis. Circulation. 2006;113(6):861–866. doi: 10.1161/CIRCULATIONAHA.105.552844
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853. doi: 10.1161/01.cir.90.2.844
- Mohler ER. Mechanisms of aortic valve calcification. Am. J. Cardiol. 2004;94(11):1396–1402. doi: 10.1016/j.amjcard.2004.08.013
- Shahi CN, Ghaisas NK, Goggins M, et al. Elevated levels of circulating soluble adhesion molecules in patients with nonrheumatic aortic stenosis. Am. J. Cardiol. 1997;79(7):980–982. doi: 10.1016/s0002-9149(97)00027-1
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 1999;19(5):1218–1222. doi: 10.1161/01.atv.19.5.1218
- Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 2000;9(5):281–286. doi: 10.1016/s1054-8807(00)00043-0
- O’Brien KD, Shavelle DM, Caulfield MT, et al. Association of Angiotensin-Converting Enzyme With Low-Density Lipoprotein in Aortic Valvular Lesions and in Human Plasma. Circulation. 2002;106(17):2224–2230. doi: 10.1161/01.CIR.0000035655.45453.D2
- Pohle K, Otte M, Mäffert R, et al. Association of cardiovascular risk factors to aortic valve calcification as quantified by electron beam computed tomography. Mayo Clin. Proc. 2004;79(10):1242–1246. doi: 10.4065/79.10.1242
- Wong ND, Sciammarella M, Arad Y, et al. Relation of thoracic aortic and aortic valve calcium to coronary artery calcium and risk assessment. Am. J. Cardiol. 2003;92(8):951–955. doi: 10.1016/s0002-9149(03)00976-7
- Fox CS, Vasan RS, Parise H, et al. Mitral Annular Calcification Predicts Cardiovascular Morbidity and Mortality. Circulation. 2003;107(11):1492–1496. doi: 10.1161/01.CIR.0000058168.26163.BC
- Tenenbaum A, Fisman EZ, Pines A, et al. Gender paradox in cardiac calcium deposits in middle-aged and elderly patients: mitral annular and coronary calcifications interrelationship. Maturitas. 2000;36(1):35–42. doi: 10.1016/s0378-5122(00)00120-1
- Sugihara N, Matsuzaki M. The influence of severe bone loss on mitral annular calcification in postmenopausal osteoporosis of elderly Japanese women. Jpn. Circ. J. 1993;57(1):14–26. doi: 10.1253/jcj.57.14
- Elmariah S, Delaney JAC, O’Brien KD, et al. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010;56(21):1752–1759. doi: 10.1016/j.jacc.2010.05.050
- Elmariah S, Delaney JAC, Bluemke DA, et al. Associations of LV hypertrophy with prevalent and incident valve calcification: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging. 2012;5(8):781–788. doi: 10.1016/j.jcmg.2011.12.025
- Adler Y, Koren A, Fink N, et al. Association between mitral annulus calcification and carotid atherosclerotic disease. Stroke. 1998;29(9):1833–1837. doi: 10.1161/01.str.29.9.1833
- Umana E, Ahmed W, Alpert MA. Valvular and perivalvular abnormalities in end-stage renal disease. Am. J. Med. Sci. 2003;325(4):237–242. doi: 10.1097/00000441-200304000-00010
- Alfrey AC. The role of abnormal phosphorus metabolism in the progression of chronic kidney disease and metastatic calcification. Kidney Int. Suppl. 2004;(90):S13–S17. doi: 10.1111/j.1523-1755.2004.09003.x
- Jesri A, Braitman LE, Pressman GS. Severe mitral annular calcification predicts chronic kidney disease. Int. J. Cardiol. 2008;128(2):193–196. doi: 10.1016/j.ijcard.2007.05.015
- Ribeiro S, Ramos A, Brandão A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol. Dial. Transplant. 1998;13(8):2037–2040. doi: 10.1093/ndt/13.8.2037
- Correia J, Rodrigues D, da Silva AM, Sá e Melo A, Providência LA. Massive calcification of the mitral valve annulus in an adolescent with Marfan syndrome. A case report. Rev. Port. Cardiol. 2006;25(10):921–926.
- Völzke H, Haring R, Lorbeer R, et al. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis. 2010;209(2):606–610. doi: 10.1016/j.atherosclerosis.2009.10.030
- Tenenbaum A, Shemesh J, Fisman EZ, Motro M. Advanced mitral annular calcification is associated with severe coronary calcification on fast dual spiral computed tomography. Invest. Radiol. 2000;35(3):193–198. doi: 10.1097/00004424-200003000-00006
- Kizer JR, Wiebers DO, Whisnant JP, et al. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36(12):2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad
- Rodriguez CJ, Bartz TM, Longstreth WT, et al. Association of annular calcification and aortic valve sclerosis with brain findings on magnetic resonance imaging in community dwelling older adults: the cardiovascular health study. J. Am. Coll. Cardiol. 2011;57(21):2172–2180. doi: 10.1016/j.jacc.2011.01.034
- O’Neal WT, Efird JT, Nazarian S, et al. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. EP Europace. 2015;17(3):358–363. doi: 10.1093/europace/euu265
- Willens HJ, Ferreira AC, Gallagher AJ, Morytko JA. Mobile components associated with rapidly developing mitral annulus calcification in patients with chronic renal failure: review of mobile elements associated with mitral annulus calcification. Echocardiogr. 2003;20(4):363–367. doi: 10.1046/j.1540-8175.2003.03042.x
- Movahed MR, Saito Y, Ahmadi-Kashani M, Ebrahimi R. Mitral Annulus Calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc. Ultrasound. 2007;5(1):14. doi: 10.1186/1476-7120-5-14
- Vistarini N, d’Alessandro C, Aubert S, et al. Surgery for infective endocarditis on mitral annulus calcification. J. Heart Valve Dis. 2007;16(6):611–616.
- Fulkerson PK, Beaver BM, Auseon JC, Graber HL. Calcification of the mitral annulus: Etiology, clinical associations, complications and therapy. Am. J. Med. 1979;66(6):967–977. doi: 10.1016/0002-9343(79)90452-2
- Takamoto T, Popp RL. Conduction disturbances related to the site and severity of mitral anular calcification: A 2-dimensional echocardiographic and electrocardiographs correlative study. Am. J. Cardiol. 1983;51(10):1644–1649. doi: 10.1016/0002-9149(83)90202-3
- Pekdemir H, Cansel M, Yağmur J, et al. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in patients with mitral annulus calcification. J. Electrocardiol. 2010;43(4):339–343. doi: 10.1016/j.jelectrocard.2010.02.013
- Sveric KM, Platzek I, Golgor E, et al. Purposeful use of multimodality imaging in the diagnosis of caseous mitral annular calcification: a case series report. BMC Med. Imaging. 2022;22:7. doi: 10.1186/s12880-021-00725-x
- Tyebally S, Chen D, Bhattacharyya S, et al. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncology. 2020;2(2):293–311. doi: 10.1016/j.jaccao.2020.05.009
- Mayr A, Müller S, Feuchtner G. The Spectrum of Caseous Mitral Annulus Calcifications. JACC Case Rep. 2020;3(1):104–108. doi: 10.1016/j.jaccas.2020.09.039
Supplementary files