Dual-energy computed tomography for head and neck cancer
- Authors: Petrovichev V.S.1, Neklyudova M.V.1, Sinitsyn V.E.2, Nikitin I.G.1
-
Affiliations:
- Radiology Department, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia
- Lomonosov Moscow State University
- Issue: Vol 2, No 3 (2021)
- Pages: 343-355
- Section: Reviews
- Submitted: 03.03.2021
- Accepted: 15.06.2021
- Published: 15.10.2021
- URL: https://jdigitaldiagnostics.com/DD/article/view/62572
- DOI: https://doi.org/10.17816/DD62572
- ID: 62572
Cite item
Abstract
This study reviewed the head and neck cancer diagnosis publications using dual-energy computed tomography (DECT). The qualitative and quantitative analysis of the data was DECT obtained using intravenous contrast enhancement for localized tumors, which shows the importance of constructing iodine maps for obtaining additional diagnostic information. Including the article is described aspects of improving visualization of the oropharyngeal region against the background of artifacts from dental implants. Several research articles highlight the current state of the issue and the role of post-processing of “raw data” DECT, obtaining a range of monochromatic images of a tumor and other pathological changes in the head and neck region in the article. Several learned treatises were also reflected. DECT with intravenous contrast enhancement and routine computed tomography to reduce radiation exposure to patients were compared particularly due to the possibility of obtaining virtual native diagnostic images from a contrasting series of DECT volumes during post-processing. In addition, this review also includes references to works that highlight the development of DECT as the method. Finally, the physical principles underlying DECT and the prospects for the development of the method are briefly represented.
Full Text
BACKGROUND
The importance of early diagnosis in head and neck cancer stems from the pathology’s dominance in the morbidity structure of malignant neoplasms worldwide [1]. In Russia, the number of patients with newly diagnosed malignant neoplasms of the head and neck region is steadily increasing [2].
Obviously, clinical and endoscopic examinations allow for evaluation of the mucosa of the oral cavity and tongue, as well as the larynx and pharynx. However, the submucosal areas remain unprotected. Moreover, exophytic and endophytic growth can occur in squamous cell cancer, but a mixed type is more common. If a primary or recurrent tumor is found in the mucosa, the submucosal component cannot be reliably assessed (only indirectly through palpation) [3, 4]. Following chemoradiotherapy, a zone of vitreous edema develops, altering the normal anatomy, appearance, and density of the mucosa. Because of their thickening and increase in volume, the root of the tongue, parapharyngeal and paraesophageal areas, and laryngeal and laryngopharyngeal elements may cause dysphagia and dyspnea and complicate endoscopic examination [5]. After immunotherapy of head and neck squamous cell cancer, pseudoprogression is possible, particularly an increase in tumor size and extent, which requires strict dynamic control to rule out true disease progression [6, 7]. All this necessitates the use of objective dynamic monitoring methods such as computed tomography (CT) and magnetic resonance imaging (MRI). Due to a lack of scanners to cover the population and the higher cost of the study, hybrid imaging techniques (positron emission tomography or single-photon emission computed tomography combined with CT) are less accessible. In addition, the timing of hybrid diagnosis during treatment is limited. Thus, for a more reliable differentiation between inflammatory and tumor changes, at least 3 months should elapse after chemoradiotherapy and surgery [8, 9].
MRI is a useful tool for detecting head and neck tumors. The application of current basic pulse sequences resolves a variety of diagnostic issues. Thus, T1, contrast-enhanced T1-weighted images, and T2 weighted sequences and fat-suppressed sequences are the best for visualizing inflammatory changes, staging tumor lesions, and revealing developmental embryonic tumors. Vascular and perfusion sequences are used to rule out vascular malformations and assess microcirculatory parameters of tissue perfusion. Diffusion-weighted images may be useful for imaging cholesteatomas, assessing malignancy and treatment response, and detecting recurrent or residual tumors in head and neck cancers [10, 11]. Aside from the obvious advantages of the method, the time required to perform a full MRI, including various pulse sequences, is longer than CT, limiting the scanner’s throughput and the number of possible examinations within a day.
CURRENT DUAL-ENERGY IMAGING TECHNIQUES
The physical methods that underpin the interaction of X-rays with substance and provide a dual-energy scanning are the photoelectric effect, Compton effect, and Thomson scattering.
The fundamental principle of dual-energy computed tomography (DECT) is based on the fact that different anatomical structures and tissues may have the same or different density depending on the X-ray energy under which they are exposed, that is, in the range between high and low kW values. The primary advantage of using dual-energy systems is the ability to decompose the images into underlying materials. In this case, different attenuations at various X-ray energies are recorded. This reveals how much of each material is present in a particular voxel of the image and expands the possibilities for postprocessing the obtained image volumes (Figure). For example, the construction of virtual non-contrast images, subtraction of bone structures, and analysis of radiopaque stones are possible. In addition, iodine maps with isolated iodine images may be generated.
The DECT method has been known since the early days of CT, but its applications were mostly limited to determining bone mineral density [12, 13]. Recently, DECT has been introduced into clinical practice to examine patients with malignant head and neck tumors. When scanning, this method employs either scanners with two energy sources or rapid current switching on the tomograph X-ray tube.
DUAL-ENERGY TOMOGRAPHY OF THE HEAD AND NECK REGION
An early scientific paper discovered no statistically significant difference in the quality of anatomical images of the head and neck region when using dual-energy (80 and 140 kW tube currents) versus routine multispiral computed tomography (MSCT, 120 kW tube current). In addition, a lower radiation load on patients was observed in DECT [14]. Concurrently, since 2010, numerous studies have been conducted to evaluate the dual-energy computed angio-graphy possibilities of the brachycephalic arterial pathology [15–25]. According to A. Schwahofer et al. [26], the use of monoenergetic reconstructions obtained during DECT volume post processing allows to reduce artifacts from metal in the oral cavity only if the density of the latter does not exceed 4.5 g/cm3 (e.g., titanium or aluminum). This has little application in calculating total radiation dose; however, it may be useful for delineating anatomical landmarks of the area of interest when planning radiotherapy. For artifacts from dental metal with a source density of >4.5 g/cm3, only a slight reduction in artifacts was observed. The majority of the patients included had metal-containing dentures or other dental hardware with an even higher density (>10 g/cm3). Thus, monoenergetic reconstructions are not a universal tool for reducing metal artifacts in radiotherapy planning [26].
In contrast, J. Weiβ et al. [27] reported that the iterative metal artifact reduction mode allows for improved visualization of the oral cavity area and surrounding anatomical structures in the presence of dental implant artifacts. The images and diagnostic significance were assessed both qualitatively according to the Likert scale and quantitatively using Hounsfield units (HU). The study discovered that in 30 cases, using metal artifact reduction made images more informative when compared to not using this mode (3.8 ± 0.5 versus 2.6 ± 0.5, respectively; p < 0.0001). When the degree of artifacts was quantified using HU, correlated results were obtained. The findings for metal artifact reduction were significantly lower than for standard reconstruction (0.9 ± 1.6 versus 20 ± 47, respectively; p < 0.05) [27].
According to N. Groβe Hokamp et al. [28], hypo- and hyperattenuating artifacts in virtual monoenergetic images (VMI) with high kiloelectronvolt (keV) values showed increased and decreased HU values compared to conventional CT imaging (CI) (CI/VMI200 keV: −218.7/−174.4 HU, p = 0.1 and 309.8/119.2, p = 0.05, respectively). In addition, artifacts in fat decreased on VMI with high keV values (CI/VMI200 keV: 23.9/16.4, p = 0.05). Moreover, a qualitative decrease in superdense artifacts on VMI with high (≥100) keV values (e.g., CI/VMI200 keV: 2(1-3)/3(1-5), p = 0.05) was observed. Reduced artifact evidence improved imaging of the soft palate and cheeks (e.g., CI/VMI200 keV: 2(1-4)/3(1-5) and 2(1-5)/3(1-5), p ≤ 0.05). In general, VMI obtained during postprocessing of DECT volumes reduces the intensity of artifacts from dental implants and improves diagnosis of the surrounding soft tissues [28].
Another study found that using high-energy VMI reduces artifacts while weakening the visualization of iodine-containing contrast agents in tumor tissue. In general, high-energy VMI (<100 keV) as useful additional diagnostic images for assessing head and neck cancer may achieve a moderate reduction in artifacts while maintaining sufficient visualization of iodine-containing contrast agents [29]. This is supported by the study of E. Liao [30], which found that DECT improves visualization of the head, neck, and spine in the presence of artifacts from metal implants. According to some other authors, spectral CT improves radial diagnosis of malignant head and neck neoplasms [31–36].
A.M. Tawfik et al. [37] conducted a study to improve imaging of head and neck tumors. This included the assessment of images obtained with different weighting factors (0.3, 0.6, and 0.8) for 80 and 140 kW, as well as the assessment of tumor lines. The study included 35 people with malignant head and neck neoplasms who were selected from a group of 60 patients with suspected cancer. The authors compared such parameters as signal-to-noise ratio, signal attenuation measurements, and objective noise between different sets of head and neck images. The results were analyzed by two independent radiologists who assessed the following parameters on a five-point scale: lesion contours, image sharpness, and subjective noise. The scientists concluded that combining DECT diagnostic data obtained at 80 kW and 140 kW tube currents with a weighting factor of 0.6 (60% of the 80 kW data) improves the signal-to-noise ratio from the tumor focus and subjectively increases the overall image quality, including tumor borders. This weighting factor showed more diagnostic information than the 0.3 coefficient, which simulates current values on a 120 kW tube and is as close as possible to standard MSCT images [37].
According to M. Li et al. [38], using DECT to analyze material composition in conjunction with reconstructed monochromatic images has a promising potential for differential diagnosis of thyroid nodules and tumor grade clarification.
When assessing the local spread of laryngeal cancer, determination of the degree of thyroid cartilage invasion is critical. In the study of this diagnostic problem, the invasion degree was assessed on a five-point scale: true invasion began with erosion (3 points) and ended with cartilage invasion (5 points). These data were used to generate iodine maps and weighted average images. Further, weighted average images and iodine maps had 100% sensitivity, specificity, and accuracy in assessing thyroid cartilage invasion. In only one case, the weighted average images missed the complete invasion of the thyroid cartilage by squamous cell cancer of the laryngeal fold; however, the prevalence was clarified on the iodine maps [39].
The potential of the method in assessing the cartilaginous structure of the larynx was described in several other studies. In particular, R. Forghani et al. [40] noted differences in signal attenuation on VMI (≥ 95 keV) of head and neck squamous cell cancer versus unossified thyroid cartilage of the larynx. In another study, a group of scientists from Japan and the United States compared MRI and DECT in assessing the cartilaginous structure of the larynx in general and the thyroid cartilage in particular in head and neck squamous cell cancer. DECT had a higher specificity when compared to MRI. Such data were obtained for both the invasion of the entire cartilaginous structure of the larynx (84% for MRI versus 98% for DECT, p < 0.004) and the thyroid cartilage (64% versus 100%, respectively, p < 0.001). The mean area under ROC curve (0.94 vs. 0.95, p = 0.70) was calculated. The sensitivity of the methods for a similar task was not significantly different for the entire cartilaginous structure of the larynx (97% for MRI versus 81% for DECT, p = 0.16) and separately for the thyroid cartilage (100% versus 89%, respectively, p = 0.50); however, there was a tendency toward a higher sensitivity for MRI. DECT allows for the avoidance of overestimation of the extent of laryngeal cartilage invasion caused by inflammatory changes. This is achieved by employing appropriate diagnostic criteria on weighted average images and iodine maps for both ossified and unossified laryngeal cartilage. Concurrently, DECT may miss small tumor invasion of ossified laryngeal cartilages, which determines the efficiency of the method precisely in the absence of overestimation of the invasion degree of cartilaginous structure of the larynx and thus contributes to the growth of organ-preserving approaches to the treatment of laryngeal and laryngopharyngeal squamous cell cancer [41].
J.L. Wichmann et al. [42] investigated the diagnostic accuracy of a series of DECT images (80 kW and linear-mixed 120 kW) in 170 cases of various head and neck pathologies. Subsequent analyses were carried out by three independent radiologists who had been referred for clinical trials. Other data, such as images from other diagnostic modalities, were not available to the examiners. The findings were compared with medical records, CT scans, and histologic reports. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated separately for each examiner. Agreement between radiologists was assessed by using intraclass correlation coefficients. The following clinical nosologies were assigned to the diagnostic groups: squamous cell cancer (n = 107; presence/absence for primary/recurrent squamous cell cancer), lymphoma (n = 40; presence/absence for primary/recurrent lymphoma), and benign diseases (n = 23; e.g., abscess). For the 80 kW imaging series and the 120 kW line-mixed images, the cumulative sensitivity, specificity, positive predictive value, and negative predictive value were 94.8, 93.0, 95.9, and 91.1, respectively. Moreover, the results (%) for the group of patients with squamous cell cancer were extremely high (94.8/95.3, 89.1/89.1, 94.3/94.4, and 90.1/91.0); a similar pattern with lymphoma-related disease (95.0, 100.0, 100.0, and 95.2 for the 80/120 kW image series) was noted. The agreement between investigators was nearly perfect (intraclass correlation coefficients of 0.82 and 0.80; 95% CI: 0.76–0.74 and 0.86–0.85). The absorbed dose per scan length was approximately 48% lower at 80 kW when compared to standard 120 kW scans (135.5 versus 282.2 mGy/cm). The researchers concluded that low-kilovoltage CT (80 kW) has high resolution, providing good diagnostic accuracy for routine clinical practice while significantly reducing the radiation load on the patient [42].
Several other studies provided similar evidence of improved imaging of primary head and neck cancers in DECT data postprocessing with two energy sources when constructing nonlinear image mixing compared to linear image mixing at low and high energies (80/140 kW) [43]. According to S. Lam et al. [44], the optimal soft tissue imaging of the head and neck region on VMI is at 65 keV, while the optimal contrast-to-noise ratio for squamous cell cancer imaging is at 40 keV. This is because there is a difference in signal attenuation between the tumor and unaffected surrounding soft tissues (p = 0.03).
A group of scientists from China investigated the use of DECT in the detection and differential diagnosis of metastatically affected cervical lymph nodes in squamous cell head and neck cancer and other lymphoproliferative diseases. The authors concluded that optimal visualization of the affected lymph nodes was achieved on 70 keV monoenergetic images. In addition, the study design included the slope of the spectral curve of soft tissue radiability for iodine molecules depending on the kW value [45]. According to F. Fu et al. [46], virtual noncontrast images obtained in DECT postprocessing for visualization of metastatically affected cervical lymph nodes are of comparable quality to a full-scale native study. Moreover, such images have a lower effective dose of radiation to the patient when compared to the standard native study (p < 0.05). The combination of DECT with intravenous contrast enhancement and subsequent construction of virtual native images allows adequate visualization of the secondary affected lymph nodes with a lower radiation exposure to the patient [46].
Forghani (Canada) [47] compared the reconstructions and postprocessing potential of DECT with one energy source and fast switching kilovoltage (kW) on an X-ray tube and DECT with two energy sources and suggested that post processing be used for optimal imaging of soft neck tissues. Thus, virtual monoenergetic reconstructions for all neck area scans were performed at 65 and 40 keV minimum; for laryngeal tumor diagnosis, similar reconstructions and iodine mapping and virtual monoenergetic reconstructions at 95 keV (or at higher values) were used. The author proposes using all of the above, except for iodine maps, to visualize tumors of the oral cavity and oropharynx and reduce dental artifacts [47]. Further, another group of scientists provides comparable data [48].
The differential diagnosis of spectral imaging in the exclusion of residual or recurrent tumors after special treatment is given in the study of the international group of authors. Thus, according to a small cohort study by Yamauchi et al. [49], when comparing virtual monoenergetic reconstructions at 40 and 70 keV, the former allows for a better differential diagnosis of recurrent or residual tumors from benign changes caused by the performed treatment. In addition, L. Yang et al. [50] discovered differences in iodine concentration, water, and spectral curve slope in affected cervical lymph nodes from patients with various nosological diseases, including thyroid and salivary gland cancer, lymphoma, and squamous cell cancer.
M.S. May et al. [51] compared dual-energy and mono-energy (70 kW) DECT scanning with two energy sources in patients with head and neck cancer. When constructing monoenergetic reconstructions at 40 keV, the former allows for better visualization of tumor borders, whereas a 70-kW monoenergetic scanning is appropriate to reduce artifacts in the presence of metal implants in the oral cavity. A group of Canadian scientists found similar results when comparing dual-energy and standard CT scanning of patients with squamous cell head and neck cancer [52].
According to R. Forghani et al. [53–55], various DECT reconstructions combined with subsequent quantitative analysis may improve the characteristics of tissue assessment and tumor imaging, including invasion into anatomical structures, which may impact patient management. L. Yang et al. [56] used DECT to assess therapeutic response to chemoradiotherapy in two groups of patients with laryngeal and pharyngeal cancer in complete and incomplete remission. The spectral curve had a different slope depending on whether or not residual tumor was. A ROC analysis of diagnostic efficiency showed an area under the curve of 0.83. When assessing the effects for esophageal cancer radiation therapy before and after, a group of other scientists proposed that the construction of iodine maps in DECT post-processing allows them to assess iodine concentration in both cases. In addition, the effect of chemoradiation therapy may be analyzed while avoiding an increased radiation dose during treatment [57].
An interesting method for assessing nasopharyngeal cancer in DECT was proposed by measuring the concentration of gold nanoparticles that were attached to folic acid as a linker. DECT has the ability to decompose the studied material, allowing for the separation and identification of different elements in the examined tissues, particularly gold nanoparticles. Actually, this diagnostic method is molecular targeted imaging of nasopharyngeal cancer cells [58].
Recently, machine learning has gained prominence in the treatment of head and neck cancer. One group of authors described the advantages of the automated multi-energy texture analysis of soft tissues of all image series compared to the analysis of individual monoenergetic reconstructions to predict metastatic lesions of loco regional lymph nodes [59]. Another group of scientists studied the DECT potential in assessing lymph nodes in the neck region in various neoplastic lesions using machine learning, including lymphoproliferative diseases [60]. This research avenue is both scientific and practical in nature, and it merits further study.
CONCLUSIONS
Thus, in addition to the examination of patients with suspected head and neck cancer, DECT with intravenous bolus contrast provides more detailed information when compared to conventional contrast MSCT. DECT enables the decomposition of the obtained images into underlying materials, in particular, to obtain both isolated and mixed iodine maps of the area of interest, i.e., tumors and metastatically affected regional lymph nodes. This information may be of diagnostic value in dynamic examination of patients following special antitumor treatment, such as remote radiation, chemotherapy, and immunotherapy.
ADDITIONAL INFORMATION
Funding source. This study was not supported by any external sources of funding.
Competing interests. The authors declare no obvious and potential conflicts of interest related to the publication of this article.
Authors’ contribution. V.S. Petrovichev ― literature review, collection and analysis of literary sources, text writing and article editing; M.V. Neklyudova ― review of literature, preparation and writing of the text of the article; V.E. Sinitsyn ― literature review, collection and analysis of literary sources, preparation and editing of the article; I.G. Nikitin ― collection and analysis of literary sources, article editing. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgements. The authors express their gratitude to Nataliya G. Pokrovskaya for support in stylistic editing of the article text.
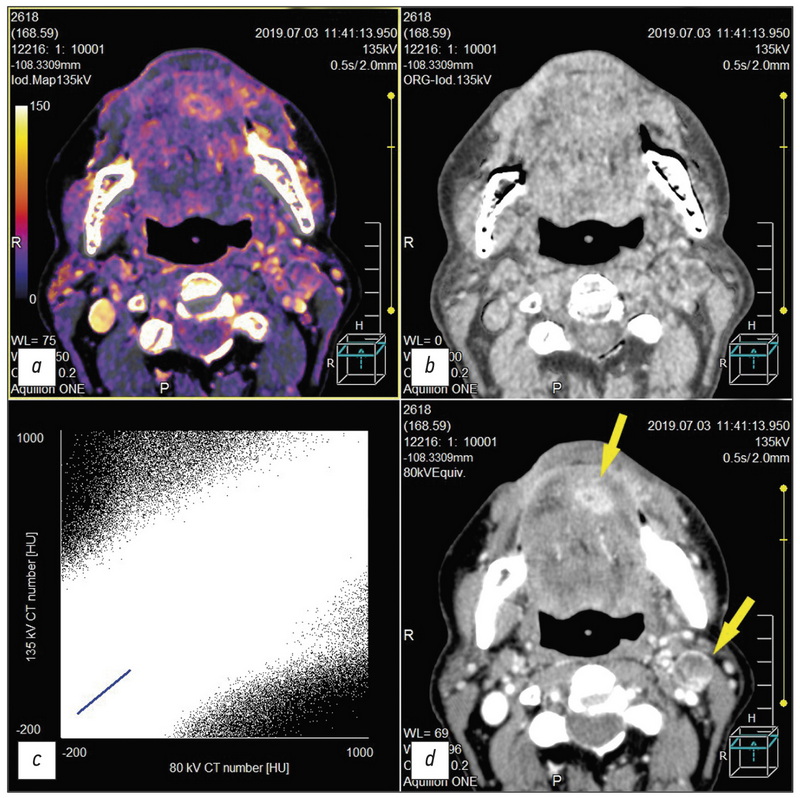
Fig. Dual-energy computed tomography after chemo radiotherapy for squamous cell cancer of the mouth floor that had spread to the tongue. Residual tumor on the lower surface of the tongue and metastasis with decay to the submandibular lymph node on the left (yellow arrows). a, iodine map; b, virtual non-contrast image; с, pixel distribution plot for an individual slice between high and low kV values; d, monochromatic image with a low value equal to 80 kV
About the authors
Victor S. Petrovichev
Radiology Department, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia
Email: petrovi4ev@gmail.com
ORCID iD: 0000-0002-8391-2771
SPIN-code: 7730-7420
MD, Cand. Sci. (Med.)
Russian Federation, 3 Ivan’kovskoe shosse, 125367, MoscowMarina V. Neklyudova
Radiology Department, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia
Email: mneklyudova@med-rf.ru
ORCID iD: 0000-0003-4224-2975
SPIN-code: 7450-6800
MD, Cand. Sci. (Med.)
Russian Federation, 3 Ivan’kovskoe shosse, 125367, MoscowValentin E. Sinitsyn
Lomonosov Moscow State University
Email: vsini@mail.ru
ORCID iD: 0000-0002-5649-2193
SPIN-code: 8449-6590
MD, Dr. Sci. (Med.), Professor
Russian Federation, MoscowIgor G. Nikitin
Radiology Department, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia
Author for correspondence.
Email: igor.nikitin.64@mail.ru
ORCID iD: 0000-0003-1699-0881
SPIN-code: 3595-1990
MD, Dr. Sci. (Med.), Professor
Russian Federation, 3 Ivan’kovskoe shosse, 125367, MoscowReferences
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492
- Socially significant diseases of the Russian population in 2018. Statistical materials. Moscow; 2019. Р. 15–17. (In Russ).
- Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(2 Suppl):S1–S30. doi: 10.1177/0194599817722550
- Mannelli G, Cecconi L, Gallo O. Laryngeal preneoplastic lesions and cancer: challenging diagnosis. Qualitative literature review and meta-analysis. Critical Reviews in Oncology Hematology. 2016;106:64–90. doi: 10.1016/j.critrevonc.2016.07.004
- Hinther A, Samson N, Lau H, et al. Volumetric changes in pharyngeal structures following head and neck cancer chemoradiation therapy. The Laryngoscope. 2020;130(3):597–602. doi: 10.1002/lary.28164
- Baxi SS, Dunn LA, Burtness BA. Amidst the excitement: A cautionary tale of immunotherapy, pseudoprogression and head and neck squamous cell carcinoma. Oral Oncology. 2016;62:147–148. doi: 10.1016/j.oraloncology. 2016.10.007
- Szturz P, Vermorken JB. Immunotherapy in head and neck cancer: aiming at EXTREME precision. BMC Med. 2017;15(1):110. doi: 10.1186/s12916-017-0879-4
- Abgral R, Querellou S, Potard G, et al. Does 18f-fdg pet/ct improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? Journal of Nuclear Medicine. 2008;50(1):24–29. doi: 10.2967/jnumed.108.055806
- Greven KM, Williams DW, Keyes JW, et al. Positron emission tomography of patients with head and neck carcinoma before and after high dose irradiation. Cancer. 1994;74(4):1355–1359. doi: 10.1002/1097-0142(19940815)74:4<1355::aid-cncr2820740428>3.0.co;2-i
- Widmann G, Henninger B, Kremser C, Jaschke W. MRI sequences in head & neck radiology – state of the art. Fortschr Röntgenstr. 2017;189(05):413–422. doi: 10.1055/s-0043-103280
- Dai YL, King AD. State of the art MRI in head and neck cancer. Clinical Radiology. 2018;73(1):45–59. doi: 10.1016/j.crad.2017.05.020
- Genant HK, Boyd D. Quantitative bone mineral analysis using dual energy computed tomography. Investigative Radiology. 1977;12(6):545–551. doi: 10.1097/00004424-197711000-00015
- Raymakers JA, Hoekstra O, van Putten J, et al. Fracture prevalence and bone mineral mass in osteoporosis measured with computed tomography and dual energy photon absorptiometry. Skeletal Radiol. 1986;15(3):191–197. doi: 10.1007/BF00354059
- Tawfik AM, Kerl JM, Razek AA, et al. Image quality and radiation dose of dual-energy ct of the head and neck compared with a standard 120-kvp acquisition. AJNR Am J Neuroradiol. 2011;32(11):1994–1999. doi: 10.3174/ajnr.A2654
- Deng K, Liu C, Ma R, et al. Clinical evaluation of dual-energy bone removal in CT angiography of the head and neck: comparison with conventional bone-subtraction CT angiography. Clinical Radiology. 2009;64(5):534–541. doi: 10.1016/j.crad.2009.01.007
- Lell MM, Kramer M, Klotz E, et al. Carotid computed tomography angiography with automated bone suppression: a comparative study between dual energy and bone subtraction techniques. Investigative Radiology. 2009;44(6):322–328. doi: 10.1097/RLI.0b013e31819e8ad9
- Thomas C, Korn A, Krauss B, et al. Automatic bone and plaque removal using dual energy CT for head and neck angiography: Feasibility and initial performance evaluation. European Journal of Radiology. 2010;76(1):61–67. doi: 10.1016/j.ejrad.2009.05.004
- Lell MM, Hinkmann F, Nkenke E, et al. Dual energy CTA of the supraaortic arteries: Technical improvements with a novel dual source CT system. European Journal of Radiology. 2010;76(2):e6–e12. doi: 10.1016/j.ejrad.2009.09.022
- Chen Y, Xue H, Liu W, et al. [Dual-energy computed tomographic angiography of head and neck arteries with different contrast material doses in second generation dual-source computed tomography system]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32(6):628–633. doi: 10.3881/j.issn.1000.503X.2010.06.008
- Korn A, Fenchel M, Bender B, et al. High-pitch dual-source CT angiography of supra-aortic arteries: assessment of image quality and radiation dose. Neuroradiology. 2013;55(4):423–430. doi: 10.1007/s00234-012-1120-y
- Chen Y, Xue H, Jin Z, et al. 128-slice acceletated-pitch dual energy ct angiography of the head and neck: comparison of different low contrast medium volumes. PLoS ONE. 2013;8(11):e80939. doi: 10.1371/journal.pone.0080939
- Korn A, Bender B, Schabel C, et al. Dual-source dual-energy ct angiography of the supra-aortic arteries with tin filter. Academic Radiology. 2015;22(6):708–713. doi: 10.1016/j.acra.2015.01.016
- Kaemmerer N, Brand M, Hammon M, et al. Dual-energy computed tomography angiography of the head and neck with single-source computed tomography: a new technical (Split filter) approach for bone removal. Invest Radiol. 2016;51(10):618–623. doi: 10.1097/RLI.0000000000000290
- Ma G, Yu Y, Duan H, et al. Subtraction CT angiography in head and neck with low radiation and contrast dose dual-energy spectral CT using rapid kV-switching technique. BJR. 2018;20170631. doi: 10.1259/bjr.20170631
- Wu Q, Shi D, Cheng T, et al. Improved display of cervical intervertebral discs on water (Iodine) images: incidental findings from single-source dual-energy CT angiography of head and neck arteries. Eur Radiol. 2019;29(1):153–160. doi: 10.1007/s00330-018-5603-z
- Schwahofer A, Bär E, Kuchenbecker S, et al. The application of metal artifact reduction (Mar) in CT scans for radiation oncology by monoenergetic extrapolation with a DECT scanner. Zeitschrift für Medizinische Physik. 2015;25(4):314–325. doi: 10.1016/j.zemedi.2015.05.004
- Weiß J, Schabel C, Bongers M, et al. Impact of iterative metal artifact reduction on diagnostic image quality in patients with dental hardware. Acta Radiol. 2017;58(3):279–285. doi: 10.1177/0284185116646144
- Große Hokamp N, Laukamp KR, Lennartz S, et al. Artifact reduction from dental implants using virtual monoenergetic reconstructions from novel spectral detector CT. European Journal of Radiology. 2018;104:136–142. doi: 10.1016/j.ejrad.2018.04.018
- Nair JR, DeBlois F, Ong T, et al. Dual-energy ct: balance between iodine attenuation and artifact reduction for the evaluation of head and neck cancer. Journal of Computer Assisted Tomography. 2017;41(6):931–936. doi: 10.1097/RCT.0000000000000617
- Liao E, Srinivasan A. Applications of dual-energy computed tomography for artifact reduction in the head, neck, and spine. Neuroimaging Clinics of North America. 2017;27(3):489–497. doi: 10.1016/j.nic.2017.04.004
- Vogl TJ, Schulz B, Bauer RW, et al. Dual-energy ct applications in head and neck imaging. American Journal of Roentgenology. 2012;199(5 Suppl):S34–S39. doi: 10.2214/AJR.12.9113
- Srinivasan A, Parker RA, Manjunathan A, et al. Differentiation of benign and malignant neck pathologies: preliminary experience using spectral computed tomography. Journal of Computer Assisted Tomography. 2013;37(5):666–672. doi: 10.1097/RCT.0b013e3182976365
- Tawfik AM, Razek AA, Kerl JM, et al. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol. 2014;24(3):574–580. doi: 10.1007/s00330-013-3035-3
- Kuno H, Onaya H, Fujii S, et al. Primary staging of laryngeal and hypopharyngeal cancer: CT, MR imaging and dual-energy CT. European Journal of Radiology. 2014;83(1):e23–e35. doi: 10.1016/j.ejrad.2013.10.022
- Toepker M, Czerny C, Ringl H, et al. Can dual-energy CT improve the assessment of tumor margins in oral cancer? Oral Oncology. 2014;50(3):221–227. doi: 10.1016/j.oraloncology.2013.12.001
- Ginat DT, Mayich M, Daftari-Besheli L, Gupta R. Clinical applications of dual-energy CT in head and neck imaging. Eur Arch Otorhinolaryngol. 2016;273(3):547–553. doi: 10.1007/s00405-014-3417-4
- Tawfik AM, Kerl JM, Bauer RW, et al. Dual-energy CT of head and neck cancer: average weighting of low- and high-voltage acquisitions to improve lesion delineation and image quality –initial clinical experience. Investigative Radiology. 2012;47(5):306–311. doi: 10.1097/RLI.0b013e31821e3062
- Li M, Zheng X, Li J, et al. Dual-energy computed tomography imaging of thyroid nodule specimens: comparison with pathologic findings. Investigative Radiology. 2012;47(1):58–64. doi: 10.1097/RLI.0b013e318229fef3
- Kuno H, Onaya H, Iwata R, et al. Evaluation of cartilage invasion by laryngeal and hypopharyngeal squamous cell carcinoma with dual-energy CT. Radiology. 2012;265(2):488–496. doi: 10.1148/radiol.12111719
- Forghani R, Levental M, Gupta R, et al. Different spectral hounsfield unit curve and high-energy virtual monochromatic image characteristics of squamous cell carcinoma compared with nonossified thyroid cartilage. AJNR Am J Neuroradiol. 2015;36(6):1194–1200. doi: 10.3174/ajnr.A4253
- Kuno H, Sakamaki K, Fujii S, et al. Comparison of MR imaging and dual-energy CT for the evaluation of cartilage invasion by laryngeal and hypopharyngeal squamous cell carcinoma. AJNR Am J Neuroradiol. 2018;39(3):524–531. doi: 10.3174/ajnr.A5530
- Wichmann JL, Kraft J, Nöske EM, et al. Low-tube-voltage 80-kvp neck ct: evaluation of diagnostic accuracy and interobserver agreement. AJNR Am J Neuroradiol. 2014;35(12):2376–2381. doi: 10.3174/ajnr.A4052
- Scholtz JE, Hüsers K, Kaup M, et al. Non-linear image blending improves visualization of head and neck primary squamous cell carcinoma compared to linear blending in dual-energy CT. Clinical Radiology. 2015;70(2):168–175. doi: 10.1016/j.crad.2014.10.018
- Lam S, Gupta R, Levental M, et al. Optimal virtual monochromatic images for evaluation of normal tissues and head and neck cancer using dual-energy CT. AJNR Am J Neuroradiol. 2015;36(8):1518–1524. doi: 10.3174/ajnr.A4314
- Wang X, Zhao Y, Wu N, et al. [Application of single-source dual-energy spectral CT in differentiating lymphoma and metastatic lymph nodes in the head and neck]. Zhonghua Zhong Liu Za Zhi. 2015;37(5):361–366.
- Fu F, He A, Zhang Y, et al. Dua-energy virtual noncontrast imaging in diagnosis of cervical metastasis lymph nodes. J Can Res Ther. 2015;11(6):202. doi: 10.4103/0973-1482.168185
- Forghani R. Advanced dual-energy CT for head and neck cancer imaging. Expert Review of Anticancer Therapy. 2015;15(12):1489–1501. doi: 10.1586/14737140.2015.1108193
- Lam S, Gupta R, Kelly H, et al. Multiparametric evaluation of head and neck squamous cell carcinoma using a single-source dual-energy CT with fast kVp switching: state of the art. Cancers. 2015;7(4):2201–2216. doi: 10.3390/cancers7040886
- Yamauchi H, Buehler M, Goodsitt MM, et al. Dual-energy CT-based differentiation of benign posttreatment changes from primary or recurrent malignancy of the head and neck: comparison of spectral hounsfield units at 40 and 70 kev and iodine concentration. American Journal of Roentgenology. 2016;206(3):580–587. doi: 10.2214/AJR.15.14896
- Yang L, Luo D, Li L, et al. Differentiation of malignant cervical lymphadenopathy by dual-energy CT: a preliminary analysis. Sci Rep. 2016;6(1):31020. doi: 10.1038/srep31020
- May MS, Bruegel J, Brand M, et al. Computed tomography of the head and neck region for tumor staging – comparison of dual-source, dual-energy and low-kilovolt, single-energy acquisitions. Invest Radiol. 2017;52(9):522–528. doi: 10.1097/RLI.0000000000000377
- Forghani R, Kelly H, Yu E, et al. Low-energy virtual monochromatic dual-energy computed tomography images for the evaluation of head and neck squamous cell carcinoma: a study of tumor visibility compared with single-energy computed tomography and user acceptance. Journal of Computer Assisted Tomography. 2017;41(4):565–571. doi: 10.1097/RCT.0000000000000571
- Forghani R, Kelly HR, Curtin HD. Applications of dual-energy computed tomography for the evaluation of head and neck squamous cell carcinoma. Neuroimaging Clinics of North America. 2017;27(3):445–459. doi: 10.1016/j.nic.2017.04.001
- Pérez-Lara A, Forghani R. Spectral computed tomography. Magnetic Resonance Imaging Clinics of North America. 2018;26(1):1–17. doi: 10.1016/j.mric.2017.08.001
- Forghani R, Mukherji SK. Advanced dual-energy CT applications for the evaluation of the soft tissues of the neck. Clinical Radiology. 2018;73(1):70–80. doi: 10.1016/j.crad.2017.04.002
- Yang L, Luo D, Yi J, et al. Therapy effects of advanced hypopharyngeal and laryngeal squamous cell carcinoma: evaluated using dual-energy CT quantitative parameters. Sci Rep. 2018;8(1):9064. doi: 10.1038/s41598-018-27341-0
- Ge X, Yu J, Wang Z, et al. Comparative study of dual energy CT iodine imaging and standardized concentrations before and after chemoradiotherapy for esophageal cancer. BMC Cancer. 2018;18(1):1120. doi: 10.1186/s12885-018-5058-2
- Khademi S, Sarkar S, Shakeri-Zadeh A, et al. Dual-energy CT imaging of nasopharyngeal cancer cells using multifunctional gold nanoparticles. IET nanobiotechnol. 2019;13(9):957–961. doi: 10.1049/iet-nbt.2019.0067
- Forghani R, Chatterjee A, Reinhold C, et al. Head and neck squamous cell carcinoma: prediction of cervical lymph node metastasis by dual-energy CT texture analysis with machine learning. Eur Radiol. 2019;29(11):6172–6181. doi: 10.1007/s00330-019-06159-y
- Seidler M, Forghani B, Reinhold C, et al. Dual-energy CT texture analysis with machine learning for the evaluation and characterization of cervical lymphadenopathy. Computational and Structural Biotechnology Journal. 2019;17:1009–1015. doi: 10.1016/j.csbj.2019.07.004
Supplementary files