Radiation methods in the diagnosis of primary and recurrent malignant ovarian struma: A case report
- Authors: Nudnov N.V.1, Ivashina S.V.1, Aksenova S.P.1
-
Affiliations:
- Russian Scientific Center of Roentgenoradiology
- Issue: Vol 4, No 2 (2023)
- Pages: 214-225
- Section: Case reports
- Submitted: 12.04.2023
- Accepted: 16.05.2023
- Published: 12.07.2023
- URL: https://jdigitaldiagnostics.com/DD/article/view/322846
- DOI: https://doi.org/10.17816/DD322846
- ID: 322846
Cite item
Abstract
We provide a rare clinical and diagnostic observation of primary and recurring malignant ovarian struma.
Malignant struma of the right ovary was detected 2 years after surgical treatment of primary benign struma of the left ovary. Six months later, the patient was diagnosed with a disease relapse, visualized exclusively according to radioisotope research methods. In the fourth year of anticancer treatment, ultrasonography revealed recurring foci along the peritoneum. According to the ultrasound data on the pelvic peritoneum and the projection of the removed right ovary, multiple solid nodes with high blood flow were visualized. Peak systolic velocity ranged from 2 to 9 cm/s in minor lesions from 4 to 12 mm, with an RI max of 0.53. For 4 years, the patient underwent radioiodine therapy with 131I with an activity of 6.0 GBq; the patient’s condition during the treatment was satisfactory.
Full Text
INTRODUCTION
Malignant struma ovarii is a rare disease referred as monodermal teratomas and somatic-type tumors (WHO Classification of Tumours, 2020) [1].
Major contributions to the knowledge about this condition were made in the late 19th century. Richard Böttlin was the first to describe thyroid tissue in the ovary in 1889, whereas struma ovarii was first characterized by Clemens von Kalden in 1895. The term “struma ovarii colloides” came into common use thanks to Julius Robert von Meyer. At about the same time, Ludwig Pick supposed that thyroid elements in “ovarian goiter” may be transformed. Over time, the whole range of thyroid disorders was characterized for struma ovarii, including nodular goiter, toxic goiter, auto-immune thyroiditis, adenoma, and carcinoma [2, 3].
Moreover, 90%–95% of struma ovarii cases are benign, and carcinoma is very rare in its setting. Available clinical and diagnostic data on struma ovarii is primarily descriptive [3–11].
The timely diagnosis of struma ovarii plays a key role in patients of reproductive age who plan to preserve fertility because the extent of surgical treatment varies based on the histological subtype of this condition. Although laparoscopic oophorectomy or ovarian resection may be considered for benign struma ovarii, malignant struma ovarii may require the removal of the uterus and its appendages and resection of the greater omentum [8]. A study showed that the average time from the initial diagnosis of struma ovarii (both benign and malignant) to advanced malignancy ranged from 2 to 9 years [2].
Considering that ovarian goiter is a neoplasm rather than hypertrophy of the ovarian stroma, as it is the case in the thyroid gland, its dissemination and metastatic potential to other organs support the point made in the latest 2020 World Health Organization Classification of Female Genital Tumors: “Peritoneal implants well-differentiated thyroid tissue in a patient with a histologically benign struma ovarii, known as “peritoneal strumosis,” is currently considered to be a metastasis of a well-differentiated follicular carcinoma originating in the struma ovarii” [1, 12]. The 5-year survival rate of patients treated with radioactive iodine (iodine-131, 131I) was higher than that of untreated patients (94.9 vs. 64.8%) [4, 10].
Medical database (PubMed and Medline) search identified studies of ultrasound (US) or magnetic resonance imaging (MRI) patterns of malignant struma ovarii and recurrent peritoneal strumosis; however, descriptions of the disease and its perfusion features are still unclear, which was the reason for publishing this study.
CASE REPORT
Patient
This study presents the features of a case of T3cN0M0 primary recurrent malignant struma ovarii in patient R. aged 25 years.
One month after childbirth, the patient underwent a left oophorectomy for a benign struma of the left ovary and right ovarian resection for a dermoid cyst of the right ovary. She was event-free for 2 years. In 2015, diagnostic laparoscopy and biopsy of peritoneal lesions were performed for ascites and ovarian enlargement in another medical institution. The Federal State Budgetary Institution “Russian Research Center for Radiology” of the Ministry of Health of Russia (RRCR) reviewed the tissue specimen slides and revealed poorly differentiated malignant struma ovarii. In blood tests performed in 2015, thyroglobulin level was 35.156 (normal, 0–50) ng/mL; anti-thyroglobulin antibodies, 0.52 U/mL; thyroid-stimulating hormone, 0.76 ng/mL (normal, <0.2); thyroxine, 0.69 pmol/L; CA-125, 1,339.5 U/mL; human chorionic gonadotropin, 1.2 mIU/mL; and alpha fetoprotein, 2.25 IU/mL.
Investigations
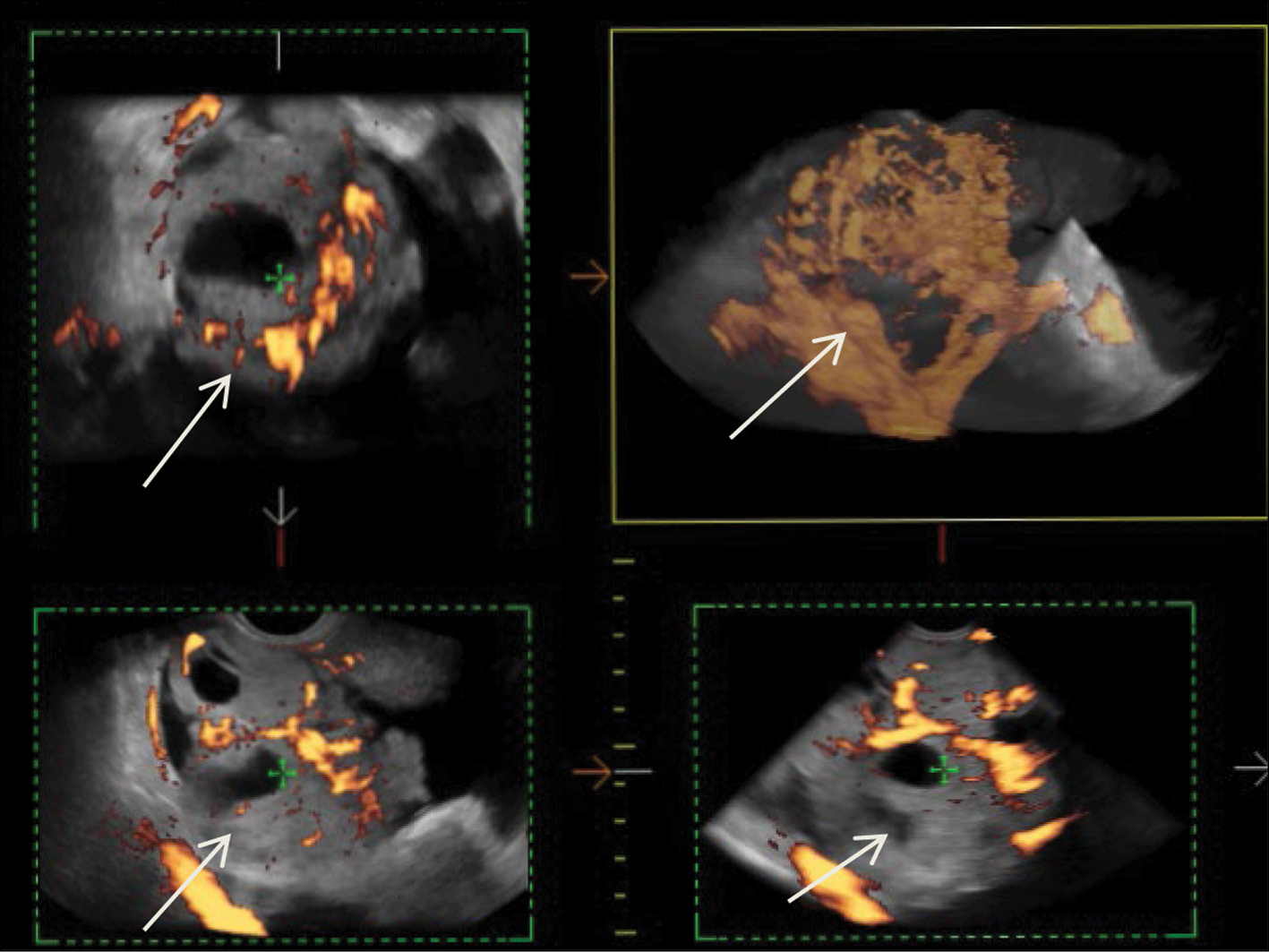
After diagnostic laparoscopy and biopsy of lesions in the pelvic peritoneum performed in another medical institution, complex ultrasonography was performed at the RRCR. An inhomogeneous isohypoechoic mass with areas of an anechoic structure of irregular and rounded shape was found in the projection of the right appendages. Pronounced pathological blood flow in the solid component of the mass, ascites, and carcinomatosis of the peritoneum of the small pelvis were also found. Figure 1 shows a 3D angiogram of the primary malignant struma ovarii on the right ovary.
Fig. 1. 3D angiography of a poorly differentiated malignant struma ovarii on the right (arrows).
eSaote Pro machine US was used; the transvaginal transducer and volume transvaginal transducer were used. Ultrasonography was performed in a standard 2D mode, and high-tech US methods were then used: power Doppler mapping, US computed tomography (CT), and 3D angiography.
Figure 2 shows uneven thickening of the pelvic peritoneum covering the anterior wall of the uterus. The structure of the blastomatously altered peritoneum of the small pelvis is isohypoechoic, with ill-defined edges on the interface with the body of the uterus.
Fig. 2. Dopplerography in the energy mode. Malignant struma ovarii. Carcinomatosis of the pelvic peritoneum anterior to the uterus (arrow). The visualized tumor lesion is 6 mm thick.
The intensity of the blood flow in the tumor lesion in the retrouterine pouch is remarkable, as well as its smoothness in the thickened (up to 5–7 mm) peritoneum of the small pelvis (Fig. 3).
Fig. 3. 3D angiography of the malignant struma lesion along the peritoneum in the retrouterine space (arrow).
In addition to US, a multiparametric MRI of the pelvic organs was performed using a high-field tomograph with a magnetic field strength of 1.5 Tesla. The MRI examination was in line with the guidelines of the European Society of Urogenital Radiology (ESUR Guidelines, 2019) and included several modes: T2-weighted imaging (T2-WI), T1-weighted imaging (T1-WI), diffusion-weighted imaging (DWI; b = 800, b = 1,400), and T1 with dynamic contrast enhancement [13].
The MRI of the malignant struma ovarii demonstrated a space-occupying predominantly solid mass of the right ovary with uneven poorly defined bumpy edges, areas of multiple cystic inclusions ranging in size from 0.7 to 3 cm with a heterogeneously increased MR signal in T1-WI, heterogeneous (from hyper- to hypo-) MR signal in T2-WI, which was consistent with colloid nodules with high-protein contents of different viscosities. Solid areas of the tumor were characterized by different rates of diffusion restriction, from 0.7 to 1.4 mm2/s × 10–3. Multiple nodular masses along the peritoneum of the small pelvis and diffuse thickening of the peritoneum were also detected. The paramagnetic accumulation in the tumor node along the peritoneum and nodes of the peritoneum was sharply increased in T1-WI, with suppression of the signal from the adipose tissue (Fig. 4). Meanwhile, sagittal post-contrast images clearly show the absence of paramagnetic accumulation in the colloid nodules of the malignant struma of the right ovary (Fig. 4f).
Fig. 4. Pelvic magnetic resonance images of patient R. with malignant struma of the right ovary: (a) T2-FS-WI in the axial plane; (b) T1-WI in the axial plane; (c) diffusion-weighted imaging (b = 1,000); (d) apparent diffusion coefficient map; (e) T1-FS-WI + contrast in the axial plane; (f) T1-FS-WI + contrast in the sagittal plane. The solid arrow indicates colloid nodules in the malignant struma of the right ovary. The dotted arrow shows lesions along the pelvic peritoneum with increased paramagnetic accumulation and restricted diffusion similar to the solid component of the primary tumor.
Three months after the extirpation of the uterus with the right appendages, omentectomy, and thyroidectomy, the US showed 5–10 mL of free fluid in the small pelvis. Based on the comprehensive US findings, the free fluid persisted for 4 years despite ongoing radioiodine therapy (131I; 6.0 GBq; a total of 11 cycles); however, no lesions were detected. The first recurrent lesion of the primary tumor along the pelvic peritoneum, visualized by US, appeared 4 years after the start of the combination therapy.
US demonstrated that the recurrent lesion of malignant struma ovarii along the pelvic peritoneum, identified in the presence of ascites, had an iso-hypoechoic structure with fuzzy contours and small size (4–12 mm). The peritoneum of the small pelvis outside the lesion was <4 mm thick; however, 3D angiography and US-CT revealed that even small blastomatous lesions were well vascularized. Blood flow was also present in the 4–5 mm thick structure of the pelvic peritoneum (Figs. 5–7).
Fig. 5. Dopplerography in the energy mode. The arrows show recurrent lesions of the malignant struma ovarii.
Fig. 6. US-CT of the recurrent lesions of the malignant struma ovarii.
Fig. 7. 3D angiography of the recurrent lesions of the malignant struma ovarii in the pelvic peritoneum in the presence of ascites.
To identify specific US signs of malignant struma ovarii, recurrent tumor lesions along the peritoneum of the malignant struma in one patient were compared with recurrent lesions of serous ovarian adenocarcinoma in 12 patients. When comparing the tumor lesions in the retrouterine space, a more pronounced neoangiogenesis was noted in the tumor lesion of the malignant struma ovarii, where the peak systolic velocity (PS) was recorded in small lesions (4–12 mm) and ranged from 2 to 9 cm/s. The maximum vascular resistivity index (RI max) was 0.53. No blood flow was noted in recurrent lesions along the pelvic peritoneum of the serous adenocarcinoma up to 9 mm, and in lesions up to 15–20 mm, PS varied from 2 to 4 cm/s or was <2 cm/s (Figs. 5 and 8).
Fig. 8. Dopplerography (energy mode) of the tumor lesion along the peritoneum in the retrouterine space in a patient with stage IIIC serous ovarian cancer.
Thus, this case demonstrated that the combined MRI findings of the thyroid tissue (colloid nodules), restricted diffusion areas, and increased paramagnetic accumulation in its solid component may indicate a malignant struma ovarii. Recurrent lesions of malignant struma, even small ones (up to 4–5 mm), are well vascularized. Malignant struma of the right ovary was detected 2 years after surgical treatment of primary benign struma of the left ovary.
The somatic condition of the patient was not aggravated by ascites and persistent recurrent tumor lesions in the small pelvis.
DISCUSSION
In this study, we analyzed US and MRI signs of primary recurrent malignant struma ovarii. Our data are generally consistent with those of individual case reports of this neoplasm in the literature. US visualizes a malignant struma ovarii as a multicystic tumor with irregular septa and heterogeneous echogenic solid components. Complex US (power Doppler mapping, US-CT, and 3D angiography) show pronounced neoangiogenesis in both primary malignant struma ovarii and its small recurrent lesions. Its characteristic feature is the so-called struma pearls, i.e., well-defined rounded solid areas, which are consistent with the colloid-rich thyroid tissue [8, 14–17].
In the presented case, the tumor did not show typical MRI signs for a malignant struma ovarii; however, the MRI pattern allows suspicion of the inclusions of colloid nodules in the thyroid tissue of the solid component of the ovarian tumor. Varying degrees of restricted diffusion were observed in the solid tumor component and lesions along the pelvic peritoneum. Extremely pronounced paramagnetic accumulation in the solid component of the tumor visualized in the post-contrast series and the absence of accumulation in the colloid nodules were pathognomonic. In the study by Gil et al. [14], MR images revealed struma ovarii as multicystic tumors with solid components, often with high T1-WI signal intensity, whereas cysts had different T2-WI signal intensities depending on fluid viscosity. Tamura et al. [17] analyzed 18 cases of struma ovarii, and their MRI study revealed a solid contrast-accumulating area that corresponded to a malignant variant of the tumor only in 54% of cases; meanwhile, restricted diffusion was established only in 11% of cases.
In the present study, restricted diffusion was heterogeneous but indicated malignant features, given the perfusion parameters in the tumor. Data similar to ours were reported by Yamauchi et al. [7]: the solid component in the malignant struma ovarii was characterized by the increased MR signal in diffusion-weighted imaging and a reduced signal in apparent diffusion coefficient maps. Thus, when compared with a pathomorphological study, the areas of the papillary carcinoma in struma ovarii corresponded to the area of true diffusion restriction in MRI.
Herein, we compared recurrent lesions of malignant struma and serous ovarian cancer. The recurrent lesions of malignant struma were similar in structure to serous ovarian cancer and had an isohypoechoic structure; however, US angiography and US-CT did not record blood flow in 4–5 mm lesions of the serous ovarian cancer. In this case, we did not register blood flow in the 4-mm lesion of serous ovarian cancer in the pelvic peritoneum. Moreover, Ranade et al. [19] reported peritoneal strumosis (struma peritonei) by US in a patient 6 years after surgical treatment of stroma ovarii, and the lesion had a mixed structure with calcifications.
Brogsitter et al. [20] reported a case of peritoneal strumosis and highlighted the role of histological examination of conditions similar to those of ovarian carcinoma. Many aspects of peritoneal strumosis remain unexplored; however, the authors suggest a connection with either rupture of mature ovarian teratoma or ovarian resection for mature ovarian teratoma. In addition, the authors noted a “slow” course of peritoneal strumosis [20]. In our case, the patient’s somatic condition did not worsen for 3 years from the detection of multiple peritoneal lesions, despite the insignificant positive effect of radioiodine therapy. In the databases studied, no up-to-date observations of recurrence of malignant struma ovarii—peritoneal strumosis—were found based on MRI data.
CONCLUSION
Preoperative diagnosis of malignant struma ovarii is quite challenging. Although struma ovarii is difficult to differentiate from ovarian adenocarcinoma, US and MRI may be instrumental for the differential diagnosis.
In summary, the combination of the thyroid tissue and areas of restricted diffusion in the solid tumor component visualized in MRI may be a sign of malignant stroma ovarii. Compared with serous ovarian cancer, small (up to 4–5 mm) recurrent lesions of malignant struma ovarii along the pelvic peritoneum are even well vascularized and can be detected using US volumetric imaging in the angio mode.
ADDITIONAL INFORMATION
Funding source. This article was not supported by any external sources of funding.
Competing interests. The authors declare that they have no competing interests.
Authors’ contribution. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work. Nudnov N.V. — concept and design of the work; agreement on the final version of the text; Ivashina S.V. — writing the text of the article, analysis of the results, preparation of illustrations; Aksenova S.P. — writing the text of the article, analysis the results, preparing illustrations; manuscript editing.
Consent for publication. Written consent was obtained from the patient for publication of relevant medical information and all of accompanying images within the manuscript in Digital Diagnostics journal.
About the authors
Nikolai V. Nudnov
Russian Scientific Center of Roentgenoradiology
Email: nvnudnov@rncrr.ru
ORCID iD: 0000-0001-5994-0468
SPIN-code: 3018-2527
MD, Dr. Sci. (Med), Professor
Russian Federation, MoscowSvetlana V. Ivashina
Russian Scientific Center of Roentgenoradiology
Email: s.ivashina@bk.ru
ORCID iD: 0000-0002-9287-2636
SPIN-code: 7829-2899
MD, Cand. Sci. (Med), Senior Research Associate
Russian Federation, MoscowSvetlana P. Aksenova
Russian Scientific Center of Roentgenoradiology
Author for correspondence.
Email: fabella@mail.ru
ORCID iD: 0000-0003-2552-5754
SPIN-code: 4858-4627
MD, Cand. Sci. (Med), Research Associate
Russian Federation, MoscowReferences
- Female Genital Tumours. WHO Classification of Tumours, 5th Edition, vol. 4. WHO Classification of Tumours Editorial Board; 2020. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020. Accessed: 15.04.2023.
- Li S, Yang T, Li X. FIGO stage IV and age over 55 years as prognostic predicators in patients with metastatic malignant struma ovarii. Front Oncol. 2020;(10):1983. doi: 10.3389/fonc.2020.584917
- Roth LM, Karseladze AI. Highly differentiated follicular carcinoma arising from struma ovarii: A report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008;27(2):213–222. doi: 10.1097/PGP.0b013e318158e958
- Ayhan S, Kilic F, Ersak B, et al. Malignant struma ovarii: From case to analysis J Obstet Gynaecol Res. 2021;47(9):3339–3351. doi: 10.1111/jog.14902
- Kanasugi M, Nishiyama H, Sanpei M, et al. Ovarian strumal carcinoid: A case report. Fukushima J Med Sci. 2023;69(1):51–55. doi: 10.5387/fms.2022-22
- Smith LP, Brubaker LW, Wolsky RJ. It does exist! Diagnosis and management of thyroid carcinomas originating in struma ovarii. Surg Pathol Clin. 2023;16(1):75–86. doi: 10.1016/j.path.2022.09.008
- Yamauchi S, Kokabu T, Kataoka H, et al. Computed tomography, magnetic resonance imaging, and positron emission tomography/computed tomography findings for the diagnosis of malignant struma ovarii: A case report. J Obstet Gynaecol Res. 2023;49(5):1456–1461. doi: 10.1111/jog.15619
- Yazawa R, Yazawa H, Fukuda K, Ohara M. Struma ovarii with massive ascites mimicking ovarian carcinoma treated with conservative laparoscopic surgery: A case report. Fukushima J Med Sci. 2023;69(1):37–43. doi: 10.5387/fms.2022-30
- Shou L, Lu J, Yang J, et al. Follicular carcinoma originating from struma ovarii: A case report. Medicine (Baltimore). 2023;102(1):e32658. doi: 10.1097/MD.0000000000032658
- Elshafie O, Hussein S, Al Kalbani M, et al. Papillary follicular variant thyroid cancer in a malignant struma ovarii: A report of a rare case. Endocrinol Diabetes Metab Case Rep. 2022;2022:21-0169. doi: 10.1530/EDM-21-0169
- Antonova IB, Fomin DK, Babaeva NA, et al. Malignant ovarian stroma. Literature review and own observation of a rare variant of the tumor. Difficult Patient. 2018;16(8-9):16–18. (In Russ).
- Giovannopoulou E, Saliaris K, Kavoura E, et al. Highly differentiated follicular carcinoma of ovarian origin: A systematic review of the literature. Curr Oncol. 2022;29(12):9105–9116. doi: 10.3390/curroncol29120712
- ResearchGate GmbH [Internet]. Alt C, Bharwani N, Brunesch L, et al.; ESUR Female Pelvis Imaging Working Group. Esur quick guide to female pelvis imaging [cite July 2019]. Available from: https://www.esur.org/fileadmin/content/2019/ESUR_2019_ESUR_Quick_Guide_to_Female_Pelvis_Imaging.pdf. Accessed: 15.04.2023.
- Gil R, Cunha TM, Rolim I. Mature cystic teratoma with high proportion of solid thyroid tissue: A controversial case with unusual imaging findings. J Radiol Case Rep. 2017;11(7):20–30. doi: 10.3941/jrcr.v11i7.2853
- Ozerskaya IA, Chekalova MA, Ivanov VA, Kazaryan GG. Ultrasound signs of ovarian tumors according to a standardized protocol. Medical Imaging. 2023;27(2):110–124. (In Russ). doi: 10.24835/1607-0763-1144
- Fujiwara S, Tsuyoshi H, Nishimura T, et al. Precise preoperative diagnosis of struma ovarii with pseud-Meigs’ syndrome mimicking ovarian cancer with the combination of 131I scintigraphy and 18F-FDG PET: Case report and review of the literature. J Ovarian Res. 2018.11(1):11. doi: 10.1186/s13048-018-0383-2
- Savelli L, Testa AC, Timmerman D, et al. Imaging of gynecologic disease (4): Clinical and ultrasound characteristics of struma ovarii. Ultrasound Obstet Gynecol. 2008;32(2):210–219. doi: 10.1002/uog.5396
- Tamura N, Murakami K, Ozaki R, et al. Current state of management of struma ovarii and preoperative imaging features: A retrospective case series study of 18 patients at a single institution. J Obstet Gynaecol Res. 2023;49(3):1007–1011. doi: 10.1111/jog.15545
- Ranade R, Rachh S, Basu S. Late Manifestation of struma peritonei and widespread functioning lesions in the setting of struma ovarii simulating highly differentiated follicular carcinoma. J Nucl Med Technol. 2015;43(3):231–233. doi: 10.2967/jnmt.114.149294
- Brogsitter C, Wonsak A, Würl K, Kotzerke J. Peritoneal strumosis. Eur J Nucl Med Mol Imaging. 2004;31(7):1057. doi: 10.1007/s00259-004-1548-3
Supplementary files